Metformin Addition Attenuates Olanzapine-Induced Weight Gain in Drug-Naive First-Episode Schizophrenia Patients: A Double-Blind, Placebo-Controlled Study
Abstract
Objective: The purpose of this study was to assess the efficacy of metformin in preventing olanzapine-induced weight gain. Method: Forty patients with schizophrenia were randomly assigned to treatment for 12 weeks with olanzapine, 15 mg/day, plus metformin, 750 mg/day (N=20), or olanzapine, 15 mg/day, plus placebo (N=20). This investigation was conducted in a double-blind fashion. Planned assessments included body weight, body mass index, proportion of patients who gained more than 7% of their baseline weight at the end of the 12-week treatment, waist circumference, waist-to-hip ratio, fasting glucose and insulin, insulin resistance index, and scores on the Scale for the Assessment of Positive Symptoms (SAPS) and Scale for the Assessment of Negative Symptoms (SANS). Results: Of the 40 patients who were randomly assigned, 37 (92.5%) completed treatments. The weight, body mass index, waist circumference, and waist-to-hip ratio levels increased less in the olanzapine plus metformin group relative to the olanzapine plus placebo group during the 12-week follow-up period. The insulin and insulin resistance index values of the olanzapine plus placebo group increased significantly at weeks 8 and 12. In contrast, the insulin and insulin resistance index levels of the olanzapine plus metformin group remained unchanged. Significantly fewer patients in the olanzapine plus metformin group relative to patients in the olanzapine plus placebo group increased their baseline weight by more than 7%, which was the cutoff for clinically meaningful weight gain. There was a significant decrease in SAPS and SANS scores within each group from baseline to week 12, with no between-group differences. Metformin was tolerated well by all patients. Conclusions: Metformin was effective and safe in attenuating olanzapine-induced weight gain and insulin resistance in drug-naive first-episode schizophrenia patients. Patients displayed good adherence to this type of preventive intervention.
Weight gain and glucose metabolism dysfunction have long been recognized as the side effect of antipsychotic drugs (1 – 2) . According to a recent study, 78.8% of patients receiving antipsychotics increased their baseline weight by more than 7% (3) . This antipsychotic side effect has lately become a major concern in the treatment of psychosis because weight gain and glucose metabolism dysfunction not only influence compliance with drug treatment but also inevitably associate with substantial morbidity (diabetes, hypertension, and cardiovascular disease) and mortality (4 , 5) .
Antipsychotics vary in their propensity to induce weight gain; clozapine and olanzapine produce the most weight gain (6 , 7) . Evidence shows that olanzapine induces weight gain in patients observed at least for 1 year (8) . The pattern of weight gain over time varies among agents, but it generally appears to be consistent with gradual deceleration (9 , 10) . As a result, it has been suggested that young patients previously unexposed to antipsychotic medication are particularly vulnerable to this antipsychotic-induced weight gain (11 , 12) .
Despite these long-recognized side effects of antipsychotic medications, there is a relative dearth of interventions to control weight gain. A variety of pharmacological agents have been studied in the efforts to reverse weight gain induced by antipsychotics. Typically, only modest weight gain reductions have been reported, and current evidence is insufficient to support any particular pharmacological approach (13 , 14) . Very few prevention-oriented studies have been conducted. A small trial of famotidine was not effective in preventing or attenuating weight gain in olanzapine-treated first-episode schizophrenia patients (15) . A pilot study found nutritional counseling and initiation of a personal exercise program to be effective in preventing weight gain, but these approaches alone are seldom effective in producing short-term weight reduction (16) .
Metformin is a hepatic-selective insulin sensitizer. Metformin reduces weight, blood glucose, insulin, and hemoglobin A1c (HbA1c) levels in obese nondiabetic adults (17 – 19) . Klein et al. recently found that metformin therapy is safe and effective in abrogating weight gain and decreases insulin sensitivity and abnormal glucose metabolism resulting from the treatment of children and adolescents with olanzapine (20) . Therefore, we hypothesized that metformin might prove useful in preventing the progression to obesity or glucose metabolic dysfunction in patients treated with olanzapine. To this end, we conducted a double-blind, placebo-controlled randomized trial.
Because young and previously untreated patients seem to be particularly vulnerable to olanzapine-induced weight gain (11 , 12) , we assessed the efficacy of metformin in preventing olanzapine-induced weight gain in drug-naive first-episode patients with schizophrenia.
Method
Subjects
The participants were recruited from the Mental Health Institute of the Second Xiangya Hospital, Central South University, P.R. China. Participants were patients age 18–50 years referred for their first psychotic episode of schizophrenia diagnosed in accordance with criteria established in DSM-IV (21) . All patients remained hospitalized and had the same diets throughout the trial. The total caloric intake range was 7980–9250 kilojoule/day. Participants performed light to moderate exercises for at least 30 minutes per day. All patients ensured that they had not used any antipsychotics or recreational drugs for at least 3 months before enrollment. The results of physical examinations and laboratory and electrocardiogram (ECG) tests were normal. The following participants were excluded from this study: pregnant or lactating women and patients with mental retardation, addictive disorder, specific systemic diseases, or other medical conditions such as diabetes mellitus, dyslipidemia, cardiovascular diseases, and hypertension. After a complete description of the study to the subjects, written informed consent was obtained in accordance with National Health and Medical Research Council guidelines. The study was approved by the Second Xiangya Hospital Ethics Committee.
Study Design
A double-blind, placebo-controlled randomized design was used. The participants were randomly assigned to 12 weeks of olanzapine (15 mg/day at 8:00 p.m.) with either metformin (750 mg/day, 250 mg before each meal [N=20]) or placebo (three times/day, one pill before each meal [N=20]). The 750 mg/day dose of metformin was based on safety and efficacy findings for a Chinese obese, nondiabetic population (22 , 23) . Only trihexyphenidyl for extrapyramidal symptoms or lorazepam for insomnia or agitation was allowed as needed. The dose of all medications remained unchanged during the entire study period. Meals were served three times per day, and patients were not involved in a special diet or special physical exercise program for weight reduction.
Assessments
Baseline assessments included demographics, a comprehensive medical history, and physical examination, including anthropometric measurements (weight, height, and waist and hip circumference) and lab work. The Scale for the Assessment of Positive Symptoms (SAPS) (24) , Scale for the Assessment of Negative Symptoms (SANS) (25) , and Treatment Emergent Symptom Scale (26) were also used for monitoring psychiatric symptoms and adverse effects. The research nurses who performed physical exams were blind to the patients’ treatment assignment.
The lab work at baseline included fasting glucose and insulin, lactic acid, liver and renal function, blood counts, and ECG. Fasting blood samples were required, and fasting was confirmed by patients or caregivers at the time the patients had their blood drawn.
Follow-up visits occurred at 2, 4, 8, and 12 weeks after randomization. At each follow-up visit, all baseline evaluations (including physical examination, anthropometric measurements, and Treatment Emergent Symptom Scale) were repeated except SAPS and SANS, which were reevaluated at week 12 only, and lab work, which was reevaluated at weeks 4, 8, and 12.
Plasma glucose and lactic acid levels and liver and renal function were determined by enzymatic procedure applying the Boehringer Mannheim/Hitachi 714 automated chemistry analyzer. We measured serum insulin with a solid-phase radioimmunoassay. Anthropometric measurements (weight, height, and waist and hip circumference) were made after participants removed their shoes and upper garments and donned an examining gown. Waist circumference was measured at the level of the umbilicus, and hip circumference was measured at the level of the greater trochanters.
The primary outcomes included the changes of weight, body mass index (weight [kg] divided by the square of height [m 2 ]), waist circumference, waist-to-hip ratio, fasting glucose, fasting insulin, proportion of patients who gained more than 7% of their baseline body weight at 3 months, and insulin resistance index, which were calculated based on the following formula of homeostasis assessment for insulin resistance model: fasting insulin (10 3 mIU/liter)×fasting glucose (mmol/liter)/22.5 (27) . The major secondary outcomes included the changes in SAPS and SANS scores and adverse effects.
Randomization to Treatment Conditions
Participants were randomly assigned (without any restriction or stratification) through a computer-generated table to one of the two treatments (olanzapine plus metformin or olanzapine plus placebo) in blocks of four to ensure approximately equal numbers of participants within the two treatment groups. The treatment assignment (randomization) was determined after completion of all assessments and after acceptance into the study. To ensure concealment of the randomization, which was conducted independently of the investigators by a research pharmacist at a separate facility, medication was provided in coded containers containing the identical appearing pills of metformin or placebo supplied by the manufacturer.
Statistical Analysis
All analyses were conducted using the Statistical Package for Social Sciences, version 11.5 (SPSS). Continuous variables were described using summary statistics such as means and standard deviations. Categorical variables were described using frequencies and percentages. Student’s t test and chi square analysis were used as appropriate to evaluate between-group differences in baseline characteristics, changes of rating scale scores from baseline to endpoint, and changes of body weight, waist circumferences, fasting glucose and insulin, waist-to-hip ratio, body mass index, and insulin resistance index from baseline to each time point. The follow-up data were continuous data. The main strategy involved analysis of covariance (ANCOVA) with repeated measurements, with corresponding baseline values as covariates. Analysis designed to compare treatments was performed separately for treatment completers (completer analyses) and for all randomly assigned patients (intent-to-treat analyses using the last observation carried forward method). The relationship between initial body mass index and body weight changes at 12 weeks was assessed using Pearson’s correlation. The difference was considered statistically significant at p<0.05.
Results
Demographic and Basic Descriptive Data
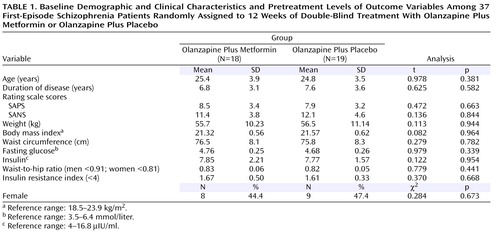
Forty patients entered the trial. Three withdrew from the study within the first 4 weeks because of lack of response (one placebo patient and two metformin patients; one man and two women). This resulted in 37 (92.7%) patients who were included in the study. The two treatment groups did not differ significantly in demographic or clinical characteristics or in pretreatment levels of the outcome variables ( Table 1 ). Their body mass indexes were within the normal range (18.5–23.9 kg/m 2 ).
The findings presented in this study summarize the analyses of completers for all assessments of the two groups among randomly assigned patients. Intent-to-treat analyses revealed similar findings.
Changes in Body Weight and Body Mass Index
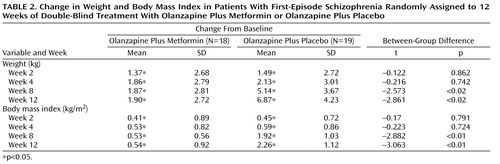
Table 2 shows descriptive data for changes in body weight and body mass index over time. Among the completers, the mean weight and body mass index values increased significantly between the two groups during weeks 2, 4, 8, and 12. At weeks 8 and 12, increases in weight and body mass index in the olanzapine plus placebo group were significantly greater relative to the olanzapine plus metformin group.
Elevated body mass index levels (>23.9 kg/m 2 ) were found in one of the 18 patients who received olanzapine plus metformin and two of the 19 patients who received olanzapine plus placebo. No significant differences were found in rates of elevated body mass index levels between the two groups.
Changes in Waist Circumference and Waist-to-Hip Ratio
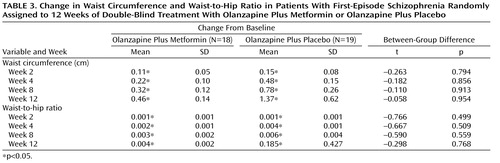
Descriptive data pertaining to changes in waist circumference and waist-to-hip ratio levels over time are displayed in Table 3 . Among completers, increases in waist circumference and waist-to-hip ratio values reached statistical significance within the two groups during weeks 2, 4, 8, and 12. Increases in waist circumference and waist-to-hip ratio levels were not significantly different between the two groups at any time point.
Changes in Fasting Glucose, Insulin, and Insulin Resistance Index
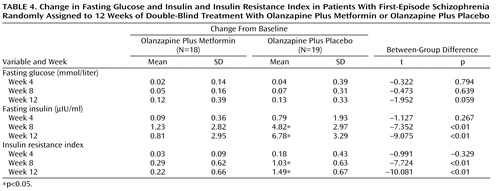
As shown in Table 4 , mean insulin and insulin resistance index levels increased significantly in the olanzapine plus placebo group during weeks 8 and 12. No significant changes in insulin and insulin resistance index levels were detected in the olanzapine plus metformin group. There were no significant changes in fasting glucose levels within the two groups. At weeks 8 and 12, increases in insulin and insulin resistance index levels of the olanzapine plus placebo group were significantly greater relative to the olanzapine plus metformin group.
Between-Group Difference in Percentage of Weight Gain
Fewer patients in the metformin group (N=3 [16.7%]), compared with the placebo group (N=12 [63.16%]), increased their initial body weight by more than 7%, the cutoff for clinically significant weight gain (28) , which was a significant difference (χ 2 =13.62, df=1, p<0.001).
ANCOVA for all assessments, excluding fasting glucose, waist circumference, and waist-to-hip ratio, found significant group effect (weight: F=9.87, df=1, 35, p<0.01; body mass index: F=8.89, df=1, 35, p<0.01; insulin: F=64.27, df=1, 35, p<0.01; insulin resistance index: F=98.21, df=1, 35, p<0.01).
There was no significant correlation between weight gain at 12 weeks and initial body mass index for either the metformin group (r=0.32, df=17, p=0.13) or the placebo group (r=0.24, df=18, p=0.28). There was also no significant correlation between the 12-week weight gain and changes in scores on SAPS and SANS for either the metformin group (r=–0.24; r=–0.12, respectively, df=18) or the placebo group (r=–0.22; r=–0.16, respectively, df=19).
Between-Group Difference in SAPS and SANS Scores
There was a significant decrease in SAPS scores within each group from baseline to week 12 (olanzapine plus metformin: 8.53 [SD=3.42] versus 2.17 [SD=1.21], t=2.37, p<0.05; olanzapine plus placebo: 7.94 [SD=3.26] versus 2.08 [SD=1.14], t=2.24, p<0.05) and in SANS scores (olanzapine plus metformin: 11.43 [SD=3.82] versus 4.16 [SD=2.03], t=2.19, p<0.05; olanzapine plus placebo: 12.14 [SD=4.63] versus 4.21 [SD=2.11], t=2.21, p<0.05). No between-group differences were found in changes from baseline in either SAPS (t=0.73, p=0.52) or SANS scores (t=0.51, p=0.64).
Metformin was well tolerated by all patients. Two of the 18 (11.1%) patients in the olanzapine plus metformin group and two of the 19 (10.5%) patients in the olanzapine plus placebo group reported nausea, which resolved within the first 1 to 7 days of therapy (χ 2 =0.12, df=1, p=0.71). No extrapyramidal symptoms were reported. No patients used trihexyphenidyl. Serum lactic acid, liver and renal function tests, and ECG examination remained normal in all patients throughout the study, and there were no episodes of vomiting or lactic acidosis.
Discussion
This randomized placebo-controlled study represents an attempt to explore the effectiveness of metformin addition to prevent antipsychotic-induced weight gain in first-episode schizophrenia patients.
In this study, we found statistically significant increases in the mean weight, body mass index, waist circumference, and waist-to-hip ratio levels within the two study groups at weeks 2, 4, 8, and 12. The increased levels of weight and body mass index were significantly less in the olanzapine plus metformin group compared with the olanzapine plus placebo group. Accordingly, significantly fewer patients in the olanzapine plus metformin group relative to the olanzapine plus placebo group increased their baseline weight by more than 7%, which was considered the cutoff for clinically meaningful weight gain. These findings show that olanzapine was associated with substantial weight gain in schizophrenia patients beginning in the second week of antipsychotic treatment and that the addition of metformin was effective in lessening olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients. Klein et al. recently reported the same findings in their study of effects of metformin in children and adolescents treated with olanzapine (20) . Our findings are also consistent with previous studies of metformin in obese nondiabetic adults. For example, metformin reduced the rates of weight gain and body fat accumulation in nondiabetic adults (29 , 30) . Preliminary short-term studies have suggested that the effects of metformin on body weight and body mass index may be mediated in part by reductions in food intake (29 , 30) .
We also found that the mean fasting insulin and insulin resistance index values of the olanzapine plus placebo group increased significantly at weeks 8 and week 12. However, there were no significant changes in fasting insulin and insulin resistance index levels in the olanzapine plus metformin group during the study. These findings suggest that olanzapine increases fasting insulin levels and insulin resistance, but metformin prevents the insulin resistance induced by olanzapine by increasing insulin sensitivity. Such effects have been reported in other studies (20 , 29 – 31) . Reduction in fasting insulin in patients treated with metformin may result primarily from increasing tissue sensitivity to insulin and reducing hepatic glucose output (32 , 33) . Fasting glucose levels did not change within the two groups during the study. It is possible that the duration of our study was too short to detect small changes in glucose that might accrue with time by olanzapine, and thus metformin did not show effects on normal glucose levels.
The addition of metformin had no adjunctive clinical effect on both positive (SAPS) and negative (SANS) symptoms in the first-episode olanzapine-treated schizophrenia patients. The fact that 92.5% follow-up was achieved supports the good adherence of the sample. There were only minor and transient side effects associated with metformin and olanzapine therapy in all patients. Plasma lactic acid levels, liver and renal function tests, and ECG examination remained normal throughout the course of the trial, and there were no episodes of lactic acidosis and no treatment-emergent extrapyramidal symptoms. Nearly all cases of lactic acidosis have occurred in patients with underlying renal or atherosclerotic cardiovascular disease, liver disease, or alcohol abuse (34 , 35) . Patients with ketosis-prone diabetes or underlying renal, hepatic, or cardiopulmonary disease should not be given metformin. Long-term therapy with metformin is associated with decreased intestinal absorption of vitamin B 12 and folate. In adults, the reductions in plasma B 12 and folate are rarely clinically important and are reversed upon discontinuation of the drug (36) . We did not measure serum B 12 and folate levels but there was no decline in hemoglobin during therapy.
To our knowledge, the present study is the first to display the effectiveness of metformin addition as a feasible treatment to prevent weight gain and its consequences in drug-naive first-episode schizophrenia patients. The addition of metformin was initiated before the presence of weight gain, and thus the aim of the intervention was to minimize olanzapine-induced weight gain before it appeared, which seems to be a more practical intervention strategy.
There were some limitations to this study. First, the duration of the study was short, and we did not follow up the changes of weight and body mass index after 12 weeks. It was unclear whether the positive effects of metformin would be substantial over long periods. However, the literature indicates that the majority of weight gain occurs during the first 12 weeks of antipsychotic treatment (37 , 38) . For olanzapine treatment, the rate of weight gain appears to be most rapid during the first 12 weeks and tends to plateau after approximately 39 weeks of treatment (39) . During the first 3 months, approximately 70% of weight gain occurs (39 , 40) . Some studies have found that medication addition may lead to short-term weight-gain control, but a regular exercise regimen may be required to maintain the long-term achievements (41) . While this study was not designed to address these long-term issues, such interventions should be explored in longer follow-up studies.
Second, we did not address whether behavioral and nutrition intervention with metformin could control the patients’ body weight more effectively. In a study of patients who had gained more than 10% of their body weight during antipsychotic treatment, we found that metformin and a lifestyle intervention had additive effects on weight loss (42) .
The third limitation was the restricted diet that our inpatient subjects were given. Therefore, it was not clear whether the same results would be seen in outpatients.
Finally, we did not examine olanzapine-metformin pharmacokinetic interactions. There are no likely metabolic interactions with metformin because this compound is not metabolized and does not inhibit the metabolism of other drugs.
According to our results, it is possible to attenuate antipsychotic-induced weight gain in drug-naive young patients with metformin, and metformin addition was safe and well tolerated by olanzapine-treated patients. Nonetheless, clinicians need to be aware of all potential adverse effects of metformin. Patients can also be treated with antipsychotics other than olanzapine, with lower potential to cause weight gain.
1. Kamran A, Doraiswamy PM, Jane JL, Hammett EB, Dunn L: Severe hyperglycemia associated with high doses of clozapine. Am J Psychiatry 1994; 151:1359Google Scholar
2. Allison DB, Mentore JL, Heo M, Chandler LP: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156:1686–1696Google Scholar
3. Mario AJ, Cesar GB, Jose VB, Rocio PI, Obdulia MG, Teresa PP, Mari RB, Benedicto CF: Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: a randomized controlled trial. J Clin Psychiatry 2006; 67:1253–1260Google Scholar
4. Newcomer JW: Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005; 19(suppl 1):1–93Google Scholar
5. Hagg S, Joelsson L, Mjorndal T: Prevalence of diabetes and impaired glucose tolerance in patients treated with clozapine compared with patients treated with conventional depot neuroleptic medications. J Clin Psychiatry 1998; 59:294–299Google Scholar
6. Wu RR, Zhao JP, Liu ZN, Zhai JG, Guo XF, Guo WB, Tang JS: Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology 2006; 186:572–578Google Scholar
7. Meyer JM: A retrospective comparison of lipid, glucose, and weight changes at one year between olanzapine and risperidone treated inpatients, in Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology. Nashville, Tenn, ACNP, 2000Google Scholar
8. Ganguli R: Weight gain associated with antipsychotic drugs. J Clin Psychiatry 1999; 60(suppl 2)10:20–24Google Scholar
9. Taylor D, McAskill R: Atypical antipsychotics and weight gain: a system review. Acta Psychiatr Scand 2000; 101:416–432Google Scholar
10. Marder S, Meibach RC: Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994; 151:825–835Google Scholar
11. Kinon MJ, Basson BR, Tollefson GD: Effect of long-term olanzapine treatment on weight change in schizophrenia. Schizophr Res 2000; 41(suppl 1):54Google Scholar
12. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ: Olanzapine treatment of children, adolescents and adults with pervasive developmental disorder: open-label pilot study. J Clin Psychopharmacol 1999; 19:37–44Google Scholar
13. Hester EK, Thrower MR: Current option in the management of olanzapine-associated weight gain. Ann Pharmacother 2004; 39:304–310Google Scholar
14. Henderson DC, Copeland PM, Daley TB, Borba CP, Cather C, Nguyen DD, Louie PM, Evins AE, Freudenreich O, Hayden D, Goff DC: A double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. Am J Psychiatry 2005; 162:954–962Google Scholar
15. Poyurovsky M, Tal V, Maayan R, Gil-Ad I, Fuchs C, Weizman A: The effect of famotidine addition on olanzapine-induced weight gain in first-episode schizophrenia patients: a double-blind placebo-controlled pilot study. Eur Neuropsychopharmacol 2004; 14:332–336Google Scholar
16. Evans S, Newton R, Higgins S: Nutritional intervention to prevent weight gain in patients commenced on olanzapine: a randomized controlled trial. Aust N Z J Psychiatry 2005; 39:479–486Google Scholar
17. Bailey CJ, Turner RC: Metformin. N Engl J Med 1996; 334:574–579Google Scholar
18. Nestler JE, Beer NA, Jakubowicz DJ, Beer RM: Effects of a reduction in circulating insulin by metformin on serum dehydroepiandrosterone sulfate in nondiabetic men. J Clin Endocrinol Metab 1994; 78:549–554Google Scholar
19. Glueck CJ, Fontaine RN, Wang P, Subbiah MT, Weber K, Illig E, Streicher P, Sieve-Smith L, Tracy TM, Lang JE, McCullough P: Metformin reduces weight, centripetal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metabolism 2001; 50:856–861Google Scholar
20. Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA: A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry 2006; 163:2072–2079Google Scholar
21. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington DC, American Psychiatric Publishing, 1994Google Scholar
22. Xinmin W, Lu L, Yulin W: Metformin, low-caloric and moderate athletics in treatment of obese. Chinese J New Drugs Clin Remedies 2003; 22:175–179Google Scholar
23. Shen Q, Hui L, Congrong G, Dajin Z: Therapeutic effect of metformin in obese population. Chinese J Endocrinol Metab 2003; 19:207–208Google Scholar
24. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
25. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
26. Guy W: ECDEU Assessment Manual for Psychopharmacology, Revised (DEW). Rockville, Md, NIMH, 1976, pp 341–350Google Scholar
27. Haffner SM, Miettinen H, Stern MP: The homeostasis model in the San Antonio Heart Study. Diabetes Care 1997; 20:1087–1092Google Scholar
28. Kanders BS, Forse RA, Blackburn GL: Methods of Obesity, in Conn’s Current Therapy. Edited by Rakel RE. Philadelphia, WB Saunders, 1991, pp 524–532Google Scholar
29. Fontbonne A, Charles MA, Juhan-Vague I: The effect of metformin on the metabolic abnormalities associated with upper-body fat distribution. Diabetes Care 1996; 19:920–926Google Scholar
30. Paolisso G, Amatao L, Eccellente R: Effect of metformin on food intake in obese subjects. Eur J Clin Invest 1998; 28:441–446Google Scholar
31. Yki-Jarvinen H, Nikkila K, Makimattila S: Metformin prevents weight gain by reducing dietary intake during insulin therapy in patients with type 2 diabetes mellitus. Drugs 1999; 58:75–82Google Scholar
32. Lachin JM, Christophi CA, Edelstein SL, Ehrmann DA, Hamman RF, Kahn SE, Knowler WC, Nathan DM: Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes 2007; 56:1153–1159Google Scholar
33. Cleasby ME, Dzamko N, Hegarty BD, Cooney GJ, Kraegen EW, Ye JM: Metformin prevents the development of acute lipid-induced insulin resistance in the rat through altered hepatic signaling mechanisms. Diabetes 2004; 53:3258–3266Google Scholar
34. Orban JC, Ghaddab A, Chatti O, Ichai C: Metformin-associated lactic acidosis. Ann Fr Anesth Reanim 2006; 25:1046–1052Google Scholar
35. Alivanis P, Giannikouris I, Paliuras C, Arvanitis A, Volanaki M, Zervos A: Metformin-associated lactic acidosis treated with continuous renal replacement therapy. Clin Ther 2006; 28:396–400Google Scholar
36. Liu KW, Dai LK, Jean W: Metformin-related vitamin B 12 deficiency. Age Ageing 2006; 35:200–201 Google Scholar
37. Tran PV, Hamilton SH, Kuntz AJ: Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol 1997; 17:407–418Google Scholar
38. Hummer M, Kemmler G, Kurz M: Weight gain induced by clozapine. Eur Neuropsychopharmacol 1995; 5:437–440Google Scholar
39. Kinon BJ, Basson MS, Gilmore LM: Long-term olanzapine treatment weight change and weight-related health factors in schizophrenia. J Clin Psychiatry 2001; 62:92–100Google Scholar
40. Addington J, Mansley C, Addington D: Weight gain in first episode psychosis. Can J Psychiatry 2003; 48:272–276Google Scholar
41. Mcguire MT, Wing RR, Klem ML: Behavioral strategies of individuals who have maintained long term weight losses. Obes Res 1997; 7:334–341Google Scholar
42. Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, He YQ, Liu YJ, Chen JD, Li LH: Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA 2008; 299:185–193Google Scholar