Facial Emotion Labeling Deficits in Children and Adolescents at Risk for Bipolar Disorder
Abstract
Objective: Research has revealed facial emotion labeling deficits in children and adolescents with bipolar disorder. To assess whether such impairments may be an endophenotype for bipolar disorder, the authors examined facial emotion identification proficiency in children who were at risk for bipolar disorder because they had a first-degree relative with the illness. Method: The facial expressions subtests of the Diagnostic Analysis of Nonverbal Accuracy scale were administered to 52 patients with bipolar disorder, 24 at-risk youths, and 78 control subjects, all 4–18 years of age. Results: Compared with the control group, both the bipolar and at-risk groups made more errors identifying facial emotions. The number of errors did not differ significantly between the bipolar and at-risk groups. Conclusions: Deficits in facial emotion labeling may be a risk marker for bipolar disorder. Further study is needed to determine the neural mechanisms involved, as well as to explore other emotional processing impairments in youths at risk for bipolar disorder and to identify genetic associations.
Because clinical phenotypes in child psychiatry are complex, the identification of endophenotypes might provide more tractable targets for genetic studies (1) . Although data suggest that attentional and memory deficits may be endophenotypes for bipolar disorder (2) , such impairments are also present in other psychiatric illnesses. Moreover, researchers have yet to identify candidate endophenotypes for bipolar disorder that involve the processing of emotional stimuli. Few studies have considered endophenotypes for bipolar disorder in children and adolescents—an important avenue of inquiry given possible opportunities for prevention.
Recent work using standardized behavioral paradigms to assess emotional processing has identified several deficits in euthymic bipolar youths (3 – 8) , including difficulty identifying facial emotions (5 , 7 , 9 , 10) . Indeed, the brain regions that mediate facial emotion processing overlap with the ventrolateral prefrontal cortex-striatum-amygdala circuit implicated in the pathophysiology of both pediatric and adult bipolar disorder (9 , 11) .
While most studies of children at risk for bipolar disorder have focused on clinical description (12) , more recent work has also examined potential neurophysiological (13) and neuropsychological (14) deficits. Research should build on this work by assessing the processing of emotional stimuli in first-degree relatives of bipolar patients. To that end, we tested the hypothesis that children with a family history of bipolar disorder would have facial emotion processing deficits similar to those seen in pediatric bipolar probands (5 , 7 , 9 , 10) . Confirmation of this hypothesis would suggest that facial emotion labeling deficits may be an endophenotype for bipolar disorder.
Method
Participants
All participants were enrolled in a study at the National Institute of Mental Health (NIMH) that was approved by the NIMH institutional review board. Parents and children gave written informed consent or assent. Participants were 4–18 years old.
Participants included patients with bipolar disorder, youths who were at risk for bipolar disorder because they had a first-degree bipolar relative, and control subjects. We conducted two sets of analyses. In our primary analyses, none of the participants were biologically related, whereas in our secondary analyses, some bipolar and at-risk participants were biologically related siblings. Our primary analyses included data from 52 patients with bipolar disorder, 24 at-risk youths, and 78 control subjects. These analyses included previously published data from 31 bipolar patients and 10 control subjects (5) . Nine of the 40 bipolar patients reported previously (5) were not used in this analysis because data from two subjects were missing for one of the subtests, and seven were excluded because they had siblings in the at-risk group. In our secondary analyses, we used all available data, including from multiple siblings from within a family. Data were from 66 bipolar patients derived from 63 independent families and 33 at-risk subjects derived from 24 independent families; we used the same control group as in the primary analysis (all biologically unrelated). In these analyses, data from 38 bipolar patients and 10 control subjects were previously published (5) .
Control subjects were drawn from the community. Bipolar patients were recruited through advertisements directed to support groups and psychiatrists. At-risk youths were eligible if they had a parent or a sibling participating in an NIMH study in which a semistructured interview confirmed a DSM-IV-TR diagnosis of bipolar I or II disorder. For parents, diagnosis was determined with either the Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient Edition (SCID-I/P) (15) or the Diagnostic Interview for Genetic Studies (16) . Bipolar siblings of at-risk participants met criteria for “narrow-phenotype” bipolar disorder, defined as having had at least one full-duration hypomanic or manic episode with abnormally elevated mood and at least three criterion B mania symptoms (17) ; diagnosis was determined with the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (18) . In addition to the structured interview, clinicians reviewed children’s medical records, consulted with treating clinicians, and performed an unstructured interview with parents of bipolar and at-risk youths over the telephone.
All participants were assessed with the K-SADS-PL to establish diagnoses. Interviewers were master’s- or doctoral-level clinicians; interrater reliability was excellent (kappa >0.9). To avoid the potential bias of the at-risk group consisting solely of resilient youths, at-risk participants with anxiety disorders or attention deficit hyperactivity disorder (ADHD) were included. However, those with current or past mood disorders were excluded because bipolar disorder can manifest first as depression, and mood disorders have been linked more consistently than anxiety or ADHD to facial emotion labeling deficits (19) . Three at-risk children were too young to be assessed with the K-SADS-PL. Control subjects had no lifetime psychiatric diagnoses.
Participants in the bipolar group met criteria for narrow-phenotype bipolar disorder. To determine mood state in bipolar and at-risk subjects at the time of testing, clinicians (with interrater reliability >0.9) administered the Children"s Depression Rating Scale (20) and the Young Mania Rating Scale (21) ; three children in the at-risk group were too young to receive mood ratings. To measure IQ, the Wechsler Abbreviated Scale of Intelligence (22) was administered to children age 6 and older and the Differential Ability Scales (23) to those under age 6.
Exclusion criteria for all groups included IQ <70, pervasive developmental disorder, unstable medical illness, or substance abuse within the past 2 months. Although the bipolar group included youths on medication, participants in the at-risk and control groups were medication free.
Procedure
We administered the child and adult facial expressions subtests of the Diagnostic Analysis of Nonverbal Accuracy scale (24) to all participants. This instrument, which has been validated in and administered to children as young as 3 years old (25) , includes standardized photographs of children (N=24) and adults (N=24) displaying expressions of happiness, sadness, anger, or fear. After viewing the photograph for 2 seconds, the participant indicates by button-press which emotion is expressed. The primary outcome variables are the number of misidentified emotions on child and adult faces.
Data Analysis
Analyses of variance (ANOVA) were used to assess group differences in age and IQ, and chi-square tests were used to assess group differences in sex distribution. Because age significantly differed between groups, it served as a covariate in the analyses of covariance (ANCOVA) used to assess between-group differences in emotion identification errors. This analysis was repeated with both age and IQ included as covariates because IQ differed between groups, although the difference fell just short of significance (p=0.06). In addition, an ANOVA was performed on subgroups matched for age because age differed significantly between groups.
Because our secondary analyses involved some observations that were nonindependent (with more than one child per family in some cases), we used a linear mixed model approach with family included as a random factor. An ANCOVA, with age included as a covariate, compared the number of emotion identification errors made by at-risk youths who had no psychiatric diagnoses to the number of errors made by the control group. T tests were used to compare the number of errors made by at-risk youths grouped by proband status (i.e., parent bipolar proband compared with sibling bipolar proband). Pearson’s correlations and t tests were used to examine relationships between performance on the facial expressions subtests and mood ratings in the bipolar and at-risk groups and between medication status and performance in the bipolar group.
Results
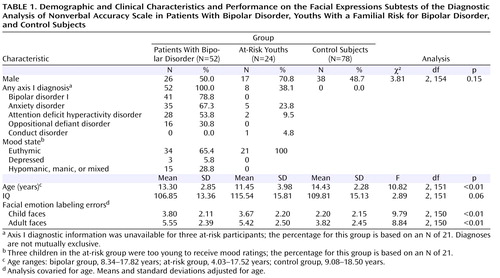
The groups differed significantly in age, but not in IQ or gender ( Table 1 ). Eight at-risk children had current or past anxiety disorders, ADHD, or conduct disorder. Most bipolar patients (78.8%) had bipolar I disorder, and the majority (90.4%) had a comorbid diagnosis. The most common comorbid diagnoses were anxiety disorders, ADHD, and oppositional defiant disorder. In the bipolar group, 41 participants (78.8%) were medicated with a mean of 3.1 medications (SD=1.3), including anticonvulsants (75.6%), atypical antipsychotics (70.7%), lithium (39.0%), antidepressants (34.1%), stimulants (29.3%), and anxiolytics (9.8%). All at-risk youths were medication free. Euthymia, defined as having a Children’s Depression Rating Scale score <40 and a Young Mania Rating Scale score ≤12, was seen in 65.4% of the bipolar patients (for all bipolar patients, mean Children’s Depression Rating Scale score=27.1 [SD=9.0] and mean Young Mania Rating Scale score=9.6 [SD=7.3]) and in all of the at-risk youths (mean Children’s Depression Rating Scale score=21.2 [SD=3.0] and mean Young Mania Rating Scale score=4.0 [SD=3.7]).
Diagnostic Analysis of Nonverbal Accuracy Scale Facial Expressions Subtests
ANCOVA revealed a group difference in emotion labeling errors in both the child and adult subtests ( Table 1 ). Bonferroni-corrected post hoc analyses showed that bipolar and at-risk youths made more errors than control subjects on both child and adult faces (bipolar group: child faces, Cohen’s d=0.75; adult faces, d=0.71; p values ≤0.01; at-risk group: child faces, d=0.68; adult faces, d=0.65; p values ≤0.02). However, the bipolar and at-risk groups did not differ from each other (child faces, d=0.06; adult faces, d=0.05; p values=1.0). Repeating this analysis with both age and IQ included as covariates yielded the same results, with differences in emotion labeling errors on both child and adult faces (adult faces: F=10.12, df=2, 149, p≤0.01; adult faces: F=8.53, df=2, 149, p≤0.01). For errors on both child and adult faces, Bonferroni-corrected post hoc analyses showed that bipolar and at-risk youths made more errors than control subjects (p values ≤0.02) but did not differ from each other. Figure 1 summarizes the total number of errors (i.e., child plus adult faces) in facial emotion identification by group. In total number of errors, 88.5% of the bipolar group and 70.8% of the at-risk group were above the mean of the control group.
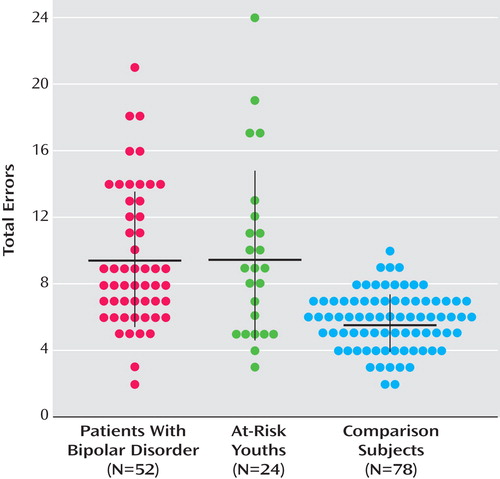
a Each dot represents a patient. The horizontal lines indicate means, and the vertical lines indicate standard deviations.
To confirm that age differences did not account for the findings, we compared subgroups matched for age (bipolar group: N=38, mean age=12.09 years [SD=2.31]; at-risk group: N=22, mean age=12.10 years [SD=3.48]; control group: N=50, mean age=13.21 years [SD=1.89]). The results mirrored those of the larger groups; between-group differences were observed in the numbers of errors for both child and adult faces (child faces: F=8.49, df=2, 107, p≤0.01; adult faces: F=10.59, df=2, 107, p≤0.01). Bonferroni-corrected post hoc analyses revealed that the bipolar and at-risk groups differed in numbers of errors on each subtest from the control group (p values ≤0.01 and ≤0.05, respectively) but not from each other.
As part of our secondary analyses, we performed a linear mixed model on the larger data set that included multiple biologically related siblings. In this analysis, family was included as a random factor and age as a covariate. Results were identical to those in the primary analyses. Again, there were between-group differences in the numbers of errors for both child and adult faces (child faces: F=8.04, df=2, 165.06, p≤0.01; adult faces F=8.22, df=2, 173, p≤0.01). Bonferroni-corrected post hoc analyses revealed that the bipolar and at-risk groups differed in number of errors on each subtest from the control group (p values ≤0.01 and ≤0.02, respectively) but not from each other. These analyses were considered secondary because the family variable was collinear with the between-group fixed factor (i.e., group) to a degree where this analytic approach could be questioned. As noted, only the bipolar and at-risk groups, but not the control group, included related individuals.
The mean number of errors on child faces was 4.0 (SD=4.5) in at-risk youths who had no psychiatric diagnoses (N=13) and 2.8 (SD=2.3) in at-risk youths with one or more psychiatric diagnoses (N=8); the corresponding means for adult faces were 4.8 (SD=3.1) and 6.1 (SD=2.0). An ANCOVA with age included as a covariate revealed that at-risk youths without psychiatric diagnoses made more errors on both child and adult faces compared with control subjects (child faces: F=9.91, df=1, 88, p≤0.01; adult faces: F=5.40, df=1, 88, p≤0.02). This suggests that the differences in facial emotion processing observed between the control group and the at-risk group were not accounted for by those at-risk individuals with ADHD or anxiety disorders.
In the at-risk group, a t test revealed no differences in the number of errors between children who were at risk by virtue of parental bipolar disorder (N=10) and those who were at risk by virtue of sibling bipolar disorder (N=14).
Pearson’s correlations revealed no association between number of errors and score on the Young Mania Rating Scale or the Children’s Depression Rating Scale in at-risk or bipolar youths. Euthymic bipolar patients (N=34) differed from control subjects in numbers of errors on both child and adult faces (p values ≤0.01). Within the bipolar group, there were no differences between unmedicated patients (mean errors: child faces=3.3 [SD=1.8]; adult faces=6.64 [SD=4.3]) and medicated patients (mean errors: child faces=4.0 [SD=2.1]; adult faces=5.3 [SD=2.6]) in performance on the facial expressions subtests, and performance and number of medications were not significantly related.
Discussion
We found that, like bipolar patients (5) , children at risk for bipolar disorder by virtue of having a parent or sibling with the illness made more errors than control subjects when identifying emotion on child and adult faces. Research is needed to ascertain whether these deficits meet the criteria for an endophenotype (1) . Data reported previously (5 , 7 , 10) indicate that deficits in facial emotion processing are state independent and are associated with bipolar disorder in the population. Data presented here demonstrate that such deficits are present in unaffected relatives of bipolar patients. Longitudinal studies are needed to ascertain whether these deficits are more common in at-risk youths who develop bipolar disorder compared with those who do not. Finally, the heritability of facial emotion processing requires further investigation. While some research indicates that facial emotion processing has a familial component in anxiety and nonbipolar mood disorders (19) , genetic studies are needed to generate heritability estimates of this function. Studies suggest that mood disorders, including bipolar disorder, may be more common in the families of patients with prepubertal-onset bipolar disorder than in the families of patients whose onset is after adolescence (26 , 27) . While our results indicate no differences in facial emotion processing in siblings of child probands compared with offspring of adult probands, we did not systematically obtain the age at illness onset for the parent probands. Therefore, it is possible that illness onset in a number of the adult probands was in childhood.
There are important limitations to this study. First, the at-risk sample was small, and although all had a first-degree relative with DSM-IV-TR bipolar disorder, not all of these relatives met criteria for narrow-phenotype bipolar disorder. Second, the narrow-phenotype criteria used to diagnose bipolar disorder are more stringent than DSM-IV-TR criteria, making our findings less generalizable. Third, the diagnosis of bipolar disorder in adult probands was generated from either the SCID-I/P or the Diagnostic Interview for Genetic Studies, although both of these interviews have been shown to generate reliable diagnoses (15 , 16) . Fourth, some at-risk youths had an anxiety disorder and/or ADHD. However, at-risk youths without diagnoses made more errors identifying facial emotions than did control subjects, which suggests that current psychopathology in the at-risk group does not account for their deficits.

1. Gottesman II, Gould TD: The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 2003; 160:636–645Google Scholar
2. Glahn DC, Bearden CE, Niendam TA, Escamilla MA: The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord 2004; 6:171–182Google Scholar
3. Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E: Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry 2004; 55:32–39Google Scholar
4. Gorrindo T, Blair RJR, Budhani S, Dickstein DP, Pine DS, Leibenluft E: Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry 2005; 162:1975–1977Google Scholar
5. McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E: Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry 2005; 162:1644–1651Google Scholar
6. Rich BA, Schmajuk M, Perez-Edgar KE, Pine DS, Fox NA, Leibenluft E: The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biol Psychiatry 2005; 58:532–539Google Scholar
7. Schenkel LS, Pavuluri MN, Herbener ES, Harral EM, Sweeney JA: Facial emotion processing in acutely ill and euthymic patients with pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2007; 46:1070–1079Google Scholar
8. Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, Brotman MA, Rich BA, Pine DS, Leibenluft E: Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2007; 46:341–355Google Scholar
9. Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E: Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA 2006; 103:8900–8905Google Scholar
10. Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, Pine DS, Ernst M, Leibenluft E: Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry 2007; 48:863–871Google Scholar
11. Pavuluri MN, O’Connor MM, Harral E, Sweeney JA: Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry 2007; 62:158–167Google Scholar
12. DelBello MP, Geller B: Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord 2001; 3:325–334Google Scholar
13. Gallelli KA, Wagner CM, Karchemskiy A, Howe M, Spielman D, Reiss A, Chang KD: N -acetylaspartate levels in bipolar offspring with and at high risk for bipolar disorder. Bipolar Disord 2005; 7:589–597 Google Scholar
14. Klimes-Dougan B, Ronsaville D, Wiggs EA, Martinez PE: Neuropsychological functioning in adolescent children of mothers with a history of bipolar or major depressive disorders. Biol Psychiatry 2006; 60:957–965Google Scholar
15. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient Edition (SCID-I/P). New York, New York State Psychiatric Institute, Biometrics Research, 2002Google Scholar
16. Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T: Diagnostic interview for genetic studies: rationale, unique features, and training: NIMH Genetics Initiative. Arch Gen Psychiatry 1994; 51:849–859Google Scholar
17. Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS: Defining clinical phenotypes of juvenile mania. Am J Psychiatry 2003; 160:430–437Google Scholar
18. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Google Scholar
19. Pine DS, Klein RG, Mannuzza S, Moulton JL 3rd, Lissek S, Guardino M, Woldehawariat G: Face-emotion processing in offspring at risk for panic disorder. J Am Acad Child Adolesc Psychiatry 2005; 44:664–672Google Scholar
20. Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R: Preliminary studies of the reliability and validity of the Children’s Depression Rating Scale. J Am Acad Child Adolescent Psychiatry 1984; 23:191–197Google Scholar
21. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Google Scholar
22. Wechsler D: Wechsler Abbreviated Scale of Intelligence. San Antonio, Tex, Psychological Corp, 1999Google Scholar
23. Elliott CD: Differential Ability Scales Administration and Scoring Manual. San Antonio, Tex, Psychological Corp, 1990Google Scholar
24. Nowicki S, Duke MP: Individual differences in the nonverbal communication of affect: the Diagnostic Analysis of Nonverbal Accuracy scale. J Nonverbal Behav 1994; 18:9–35Google Scholar
25. Nowicki S, Mitchell J: Accuracy in identifying affect in child and adult faces and voices and social competence in preschool children. Genet Soc Gen Psychol Monogr 1998; 124:39–59Google Scholar
26. Strober M, Morrell W, Burroughs J, Lampert C, Danforth H, Freeman R: A family study of bipolar I disorder in adolescence: early onset of symptoms linked to increased familial loading and lithium resistance. J Affect Disord 1988; 15:255–268Google Scholar
27. Pauls DL, Morton LA, Egeland JA: Risks of affective illness among first-degree relatives of bipolar I old-order Amish probands. Arch Gen Psychiatry 1992; 49:703–708Google Scholar