Risk and Resilience Markers in Bipolar Disorder: Brain Responses to Emotional Challenge in Bipolar Patients and Their Healthy Siblings
Abstract
OBJECTIVE: The authors previously identified depression-specific differences in brain responses to an emotional challenge in patients with bipolar and unipolar mood disorder. In this study, potential markers of bipolar risk and resilience were examined in a new cohort of lithium-responsive bipolar patients and their healthy siblings. METHOD: Changes in regional cerebral blood flow (rCBF) were measured with [15O]water positron emission tomography after induction of transient sadness in nine euthymic lithium responders and nine healthy siblings. The patterns of change in these groups were compared, and then they were contrasted with previous findings on bipolar responders to valproate. RESULTS: Common to all three groups with induced sadness were rCBF increases in the dorsal/rostral anterior cingulate and anterior insula and decreases in the orbitofrontal and inferior temporal cortices. Distinguishing the groups were decreases in the medial frontal cortex in the patients but an increase in this region in the siblings. DISCUSSION: Common changes with emotional challenge were identified in bipolar patients and their healthy siblings. These were not seen previously in healthy subjects without a family history of mood disorder, suggesting a potential marker of bipolar risk. The siblings’ unique increases in the medial frontal cortex appear to identify a compensatory response in this at-risk group, as this pattern was not seen previously in healthy subjects without depression risk factors. This differential change pattern in patients and their siblings highlights the role of the anterior cingulate and medial frontal regions in mediating resiliency and vulnerability in bipolar disorder families.
In many bipolar patients, new episodes are triggered by an exaggerated response to emotional stimuli (1, 2). Pharmacological treatment can reduce or eliminate the recurrences of the illness, and there is some evidence that response to a specific mood stabilizer, such as lithium or valproate, is linked to certain characteristics of the course of illness (3, 4). Lithium response is associated with an episodic, remitting course of illness, with “classical” episodes and low rates of comorbid conditions (5–7). Many lithium responders have histories of severe and chronic episodes before the initiation of lithium but remain stable for years after they start taking medication (5–7). The absence of clinically relevant episodes often makes them indistinguishable from their nonaffected family members.
Genetic studies indicate that bipolar disorder is highly heritable, but not all genetically predisposed individuals will manifest the illness (8, pp. 373–401). However, such unaffected individuals are known to manifest traits associated with the illness, namely pathological reactivity to emotional stress albeit without decompensation to illness.
To test this at the brain level, we measured regional cerebral blood flow (rCBF) using positron emission tomography (PET) and a previously validated acute mood challenge (9–11) in two groups of subjects: lithium-treated bipolar subjects who had remained stable for a long period and their nonbipolar, healthy siblings. We compared the results to those for a group of euthymic valproate-treated bipolar patients who had been challenged with the same mood-induction protocol in a previous study (10). We hypothesized that changes under such a mood-related stress would unmask findings in unaffected siblings that were similar to those seen in the patients, identifying sites of vulnerability to bipolar disorder. We further hypothesized that we would also find markers of resilience in the at-risk family members, evidenced by differences in cortical responses to the acute mood change between the siblings and patients.
Method
New Subjects
The study was performed at the Centre for Addiction and Mental Health, University of Toronto. Nine subjects with bipolar disorder, type I, who had been stable while receiving lithium prophylactic monotherapy for at least 3 years and their nonbipolar, healthy siblings were included in the study. These subjects were recruited from three different centers at Dalhousie University (Halifax, N.S., Canada), McMaster University (Hamilton, Ont., Canada), and the University of Toronto. They were thoroughly diagnosed by experienced clinicians (including M.A. and L.T.Y.), and the diagnoses of bipolar disorder had been made by using the Structured Clinical Interview for DSM-IV (SCID).
Most of the bipolar patients had had previous trials of medications other than lithium and had not responded to these. These patients had a clear history of illness with previous severe episodes of both mania and depression. Lithium response was defined as the absence of symptoms of depression, mania, hypomania, and mixed mania for a minimum of 36 months while the patient was receiving lithium monotherapy for bipolar illness. All patients had to have a high risk of recurrence based on the previous course of illness and had to be compliant with the medication. This was verified by review of the clinic records provided by the treating physicians. The majority of patients had been in treatment at the respective facilities for years, and many had participated in previous studies on lithium response. Each subject was seen by one of us (S.K.) for a clinical interview to assess current mood state.
The exclusion criteria for the bipolar patients comprised other axis I or II diagnoses, history of head trauma or of substance abuse, medical and neurological comorbidity, and treatment with medications other than mood stabilizers. The healthy siblings were also assessed with the SCID to exclude any axis I or II diagnosis and any current substance abuse. Siblings with medical disorders were also excluded. Written informed consent was obtained from all subjects, and the study was approved by the Centre for Addiction and Mental Health Ethics Committee.
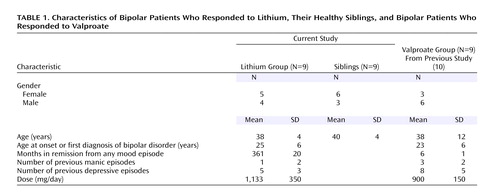
The patients’ and siblings’ characteristics are summarized in Table 1. Of note is that most lithium responders reported having had unrecognized mood episodes since their teenage years and had responded exceptionally well to lithium monotherapy, having been euthymic for approximately 3 years. All were receiving stable doses of lithium, and the mean serum level was 0.6 mmol/liter (SD=0.3). The lithium dose and level were obtained from the treating clinician at the patient’s last visit, which was scheduled shortly before the PET scan. The siblings of the lithium-treated patients had no psychiatric illnesses and no significant medical comorbidity. They were past the age of onset of bipolar disorder (Table 1).
Valproate Responders
The previously studied euthymic bipolar patients who had responded to valproate were used for a post hoc comparison with the lithium responders. The valproate responders, who were extensively described in an earlier report (10), had been euthymic for 6 months. Clinically, these patients had a more severe course of illness, including intraepisodic cycling and breakthrough episodes.
Mood Induction
Induction of transient intense sadness was performed by using a previously validated technique for mood induction (9, 11). In brief, the subjects were requested to draft a short autobiographical script describing a sad life event. Each script was tested before scanning to ensure reliability and reproducibility in inducing a sad mood. During the PET experiment, the script was projected onto a computer screen, to facilitate recall of the event while the subject lay in the scanner. The script was presented until the subject reached peak emotion. The actual scan was done 1 minute after the script was turned off. While the scan was acquired, the subject was instructed to keep his or her eyes closed and not to actively ruminate on the script content or to actively attempt to attenuate or cognitively modulate the intense negative feelings. This instruction served to reduce known activation of frontal brain regions during working memory or reappraisal tasks, as demonstrated previously (9, 12). Compliance with these instructions was assessed during the 11-minute interval between scans.
Mood was rated every 2 minutes after script presentation up to the time of the scan and immediately thereafter to document compliance with instructions. Intensity of sadness was quantified by using a self-rating Likert scale with a range of 0 (not sad) to 7 (very sad). For each subject, a scan was not performed unless the mood state reached a minimum of 6 on the rating scale. Anxiety, tension, anger, and active ruminations (thinking sad thoughts versus feeling sad thoughts) were similarly rated before and after each scan, as these emotions are known to confound the brain changes associated with sadness (9).
Scan Acquisition and Image Reconstruction
We measured rCBF by using [15O]water and PET in two behavioral states: baseline euthymia and provoked sadness. Two trials in each condition were conducted by using an alternating design, with neutral mood always preceding sad mood. All subjects were examined at the Centre for Addiction and Mental Health at the University of Toronto with the same PET machine, a GEMS/Scanditronix (Bartlett, Tenn.) 2048B camera (15 parallel slices; 6.5-mm interslice distance) using measured attenuation correction (68Ge/68Ga transmission scans). Measurement of rCBF was done by means of the bolus [15O]water technique (dose, 45 mCi of [15O]water per scan; scan initiated with bolus entry into the brain; scan duration, 60 seconds). Each subject was fitted with a customized thermoplastic face mask to minimize head movement across acquisitions. The scans were spaced a minimum of 11 minutes apart to accommodate radioactive decay to background levels as well as to allow return to behavioral baseline.
Data Analysis
Statistical analyses were performed by using SPM 99 (Wellcome Department of Cognitive Neurology, London) implemented in Matlab version 5.3 (Mathworks, Sherborn, Mass.). The data were first screened for distributional properties, outliers, and missing values. This process rejected no scans. All scans were then normalized within SPM 99 to the International Consortium for Brain Mapping (ICBM) 152 stereotactic template of the Montreal Neurological Institute, which references brain locations in three-dimensional space relative to the anterior commissure (13 , 14). MRI scans of individual study subjects were not acquired. The images were then corrected for differences in the whole-brain global mean and were smoothed by using a Gaussian kernel to a final in-plane resolution of 10 mm at full width at half maximum. Final inclusion in group analyses required further evidence of a stable baseline scan in both neutral trials. No scans were excluded because of artifacts or an incomplete return to baseline behavioral levels.
The statistical analyses used a random-effects model executed in SPM 99. Cluster significance thresholds were set at the default 50 voxels (voxel=8 mm3). On the basis of previous results of inducing sad mood in bipolar and unipolar subjects (9–11, 15), the significance thresholds for peak voxel values were set at p<0.01 for the regions we hypothesized would show significant changes—medial frontal cortex (Brodmann’s area 9/10), orbitofrontal cortex (Brodmann’s area 11), dorsal anterior cingulate (Brodmann’s area 24), dorsolateral prefrontal cortex (Brodmann’s area 9/46), and anterior insula—and at p<0.001 (uncorrected) for regions where we did not expect significant changes. Brain locations are reported as x, y, z coordinates in Montreal Neurological Institute space with approximate Brodmann areas identified by mathematical transformation of SPM 99 coordinates into Talairach space (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/). Specific analyses addressed 1) common changes across the three groups to detect a general vulnerability marker for bipolar disease, 2) lithium responders (rest versus sad) versus valproate responders (rest versus sad) to investigate differences in mood reactivity as a function of bipolar subgroup, and 3) lithium responders (rest versus sad) versus siblings (rest versus sad) to investigate the difference between illness expression and a vulnerability trait. The contrasts between the resting and sad states were performed separately for each group and were then followed by pairwise assessments of differences in change patterns between groups (valproate versus lithium group change, lithium versus sibling group change). The single-group change pattern was used to determine whether significant differences in the group analyses were due to differences in the magnitude of the same change or distinct group-specific effects. The influence of gender on the mood-induction change pattern and the stability of the change pattern between the two trials were examined by direct contrast.
Results
Behavioral Effects of Mood Induction
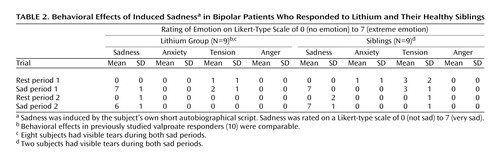
The mood-provocation protocol produced robust behavioral effects in the lithium responders and their siblings. Two siblings and eight lithium-treated patients had visible tears. Similar magnitudes of behavioral change were seen in both trials in both groups (Table 2).
In the lithium group, a maximal sad state was provoked within 20 seconds to 2 minutes after exposure to the sad script. Return to the baseline level was equally rapid. No significant anxiety was reported during any of the scans.
The siblings reported slightly higher baseline anxiety and tension (Table 2). The induction of sad mood was similar to that for the lithium group, and all subjects reached peak sadness.
All subjects remained at peak sadness throughout the scan acquisition; this was verified by postscan self-report and debriefing. All participants confirmed that they were successful in avoiding active ruminations or attempts to actively modulate their negative mood state after the script was turned off. The siblings did, however, report feeling less “overwhelmed” by the sad emotional experience than the lithium-treated patients. All subjects remained stable at follow-up 2 to 3 days later.
rCBF Changes With Sadness
Lithium group versus siblings
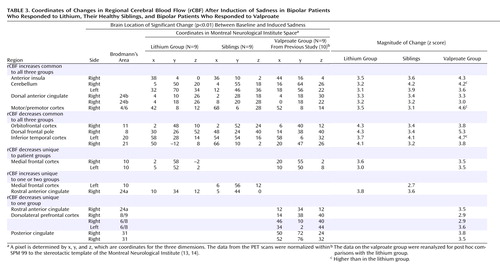
The siblings’ pattern of rCBF changes was similar to that of the lithium-treated patients: increased rCBF in the premotor cortex (Brodmann’s area 4/6), dorsal and rostral anterior cingulate (Brodmann’s area 24a/b), anterior insula, and cerebellum and decreased rCBF in the orbitofrontal cortex (Brodmann’s area 11) and inferior temporal cortex (Brodmann’s area 20/21) (Table 3, Figure 1).
Contrasts between the change patterns in the two groups revealed differences in the medial frontal cortex. Post hoc analysis showed that these differences were driven by both increases in the siblings and decreases in the lithium group (Table 3, Figure 1).
Lithium group versus valproate group
For both the lithium and valproate groups, induced sadness resulted in increases in the premotor cortex (Brodmann’s area 4/6), dorsal anterior cingulate (Brodmann’s area 24b), anterior insula, and cerebellum and decreases in the ventral medial frontal (Brodmann’s area 10), orbitofrontal cortex (Brodmann’s area 11), and inferior temporal cortex (Brodmann’s area 20/21) (Table 3, Figure 1).
Comparison of the change patterns in the lithium responders and valproate responders showed differences in the rostral anterior cingulate (Brodmann’s area 24a) and the dorsolateral prefrontal cortex. Post hoc analysis revealed that the difference in Brodmann’s area 24a was the result of both an increase in this region in the lithium group and a decrease in the valproate group. The differences in the dorsolateral prefrontal cortex were driven by decreases in the valproate group, which were absent in the lithium group. Post hoc analyses further demonstrated that the magnitude of the changes common to both groups was greater in the valproate responders in most regions (Table 3, Figure 1). There were no significant differences between men and women or between the two scans in any of the groups, although the statistical power was limited.
Discussion
Three major findings emerged from this study: 1) trait changes with induction of sad mood in the orbitofrontal cortex and dorsal anterior cingulate that were common to bipolar patients and family members at risk, 2) illness-specific changes in the medial frontal cortex that distinguished both patient groups from the at-risk siblings, and 3) changes specific to the bipolar subgroup in the dorsolateral prefrontal cortex and rostral anterior cingulate that distinguished valproate- from lithium-responsive patients.
Our findings show that the biological correlates of emotional vulnerability in both patients and siblings are decreased rCBF in the orbitofrontal cortex coupled with an increase in the dorsal anterior cingulate. Orbitofrontal hypoactivity is linked to shifts between euphoric and dysphoric mood states and the inability to differentiate between relevant and irrelevant emotional stimuli (16–18). Contiguous but more dorsal and rostral medial frontal regions are involved in mediating self-referential processing of emotionally salient stimuli and in reward assessment (19–25). The close reciprocal connection of the orbitofrontal cortex to the dorsal anterior cingulate (26) suggests that the interplay of these regions is involved in the greater vulnerability to emotional provocation. The dorsal anterior cingulate has been implicated in attention to emotional salience and in monitoring the extent of emotional response to various stimuli (27–29). Blood flow changes in these two regions in opposite directions may indicate counterregulatory mechanisms of the dorsal anterior cingulate contributing to decreased orbitofrontal functioning.
The siblings did not meet the criteria for any psychiatric diagnosis. However, our findings suggest that when emotionally provoked, siblings show changes in brain activity consistent with those of their bipolar family members. High baseline rCBF in the ventral medial frontal cortex that increased further with provoked sadness distinguished the healthy siblings from the lithium-treated patients, in whom it was decreased. Activation of the ventral medial frontal cortex is associated with emotional processing tasks, including active rethinking and reappraisal of feelings (19, 20, 30–33), and with heightened awareness of and attention to self (34, 35). Thus, the presence of increased activity in the medial frontal cortex may reflect a compensatory effect and a capacity for resilience in the siblings. As mentioned earlier, the siblings reported not having felt emotionally overwhelmed during the experiment. This might reflect the behavioral correlate of immediate activation of medial frontal regions.
The regional changes in the healthy siblings and their lithium-treated relatives differed from those previously observed in healthy subjects without any history of familial mood disorder (15). Those comparison subjects were eight healthy women (mean age=36 years, SD=6) without personal or family history of an affective disorder, who were scanned with the same mood-induction protocol under identical experimental conditions on a PET camera of the same generation as the one in this study. With sadness, these comparison subjects showed increased rCBF in the subgenual cingulate, which was not observed in either bipolar subjects or healthy siblings (9, 11); there was also an absence of any significant change in the medial frontal cortex.
There is evidence that response to a specific mood stabilizer may be linked to illness characteristics or illness subtypes (7, 36, 37). Mood lability in this context may be a predictive factor, and pharmacological stabilization of interepisodic mood swings may protect against emotional dysregulation at the cortical level. Similarly, cognitive behavior therapy leads to longer periods of remission (38, 39) by decreasing patients’ vulnerability to stress through cortical mechanisms (40).
None of the lithium-treated patients in this study had experienced a clinically significant bipolar episode for years. Only up to 40% of bipolar patients are lithium responders, but those who do respond usually do well with lithium (8, pp. 684–701; 41). Because of this observation, there has always been a debate about whether this limited but impressive response to lithium is due to its possible cortical neuroprotective effects (42). Our data cannot prove or refute this hypothesis, but it can be assumed either that lithium stabilizes pathways responsible for emotional and behavioral reactions to stressful events or that the course of illness in these highly selected bipolar patients may leave cortical circuits at least partially intact. This alternative explanation implies that our findings may represent the benign course of illness in the lithium responders, rather than a protective effect of this medication. The argument against this interpretation would be that all of these lithium responders had had long and severe episodes of illness before lithium treatment, including failed treatments with other medications in at least some cases.
In general, response to valproate has been associated with a more severe course of illness (43), including decreased cognitive functioning and functional impairment (44, 45). The regional abnormalities seen in this group are consistent with these clinical observations. With induction of sad mood, the blood flow differences between the valproate and lithium responders became more pronounced. The changes in the valproate group were more widespread in the orbitofrontal cortex, and they exhibited decreased rCBF in the dorsolateral prefrontal cortex, which was absent in the lithium group. Blood flow in the rostral anterior cingulate went in opposite directions in the two groups. The rostral anterior cingulate is involved in detecting shifts in affect and in correcting spontaneous emotional responses after provocation (46). Decreased rCBF in this region may thus lead to heightened reactivity to emotional stress. Since rCBF in the rostral anterior cingulate showed an increase in the siblings and the lithium group, decreased perfusion suggests that this region is a marker of severity of bipolar illness.
A regional change discriminating the lithium and valproate responders was the decreased activity in the dorsolateral prefrontal cortex in the valproate group and the absence of involvement of this region in the lithium group. Decreased rCBF in the dorsolateral prefrontal cortex is one of the most consistent findings in imaging studies of major depression (47–51) and has been associated with cognitive impairment (52, 53). The absence of change in the dorsolateral prefrontal cortex of the lithium responders supports the notion of partially intact cortical responses in emotionally challenging situations. The findings in the valproate group were not related to these subjects’ relatively brief remission period (6 months), which may still be considered a stage of transition out of an episode of illness, as in a previous study we clearly found differences between depressed patients and these euthymic valproate responders (10). Thus, our findings indicate that the imbalanced interplay between brain regions working in concert to modulate cortical responses, self-awareness, and the emotional state of an individual would be expected to lead to loss of control over reactions to feelings or situations with an emotional content.
In conclusion, our data provide evidence of differential cortical and cingulate responses to a transient mood in three different groups within the bipolar spectrum. While a specific pattern of change in the medial frontal cingulate did distinguish patients from their at-risk but unaffected siblings, explicit studies of cognitive mechanisms mediating this apparent cortical resilience are needed (54), as are prospective studies to determine whether failure to show such changes is a predictor of eventual illness onset.
 |
 |
 |
Received Sept. 23, 2004; revision received March 17, 2005; accepted May 13, 2005. From the Centre for Addiction and Mental Health, University of Toronto, Toronto; and the Department of Psychiatry, Dalhousie University, Halifax, N.S., Canada. Address correspondence and reprint requests to Priv. Doz. Dr. Krüger, Klinik und Poliklinik für Psychiatrie und Psychotherapie, Universitätsklinikum Carl-Gustav Carus Dresden, Fetscherstrasse 74, 01307 Dresden, Germany; [email protected] (e-mail).Supported in part by a grant from the National Alliance for Research on Schizophrenia and Depression to Dr. Alda; Dr. Krüger holds the Margaret Botterell Fellowship in Bipolar Studies.
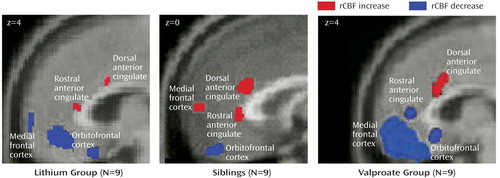
Figure 1. Frontal and Anterior Cingulate Changes in Regional Cerebral Blood Flow (rCBF) After Induction of Sadness in Bipolar Patients Who Responded to Lithium, Their Healthy Siblings, and Bipolar Patients Who Responded to Valproatea
aThe bipolar patients in the valproate group had been euthymic for 6 months and were described previously (10).
1. Fukuda K, Etoh T, Iwadate T, Ishii A: The course and prognosis of manic-depressive psychosis: a quantitative analysis of episodes and intervals. Tohoku J Exp Med 1983; 299-307Google Scholar
2. Keller MB, Lavori PW, Coryell W, Andreasen NC, Endicott J, Clayton PJ, Klerman GL, Hirschfeld RM: Differential outcome of pure manic, mixed/cycling, and pure depressive episodes in patients with bipolar illness. JAMA 1986; 255:3138–3142Crossref, Medline, Google Scholar
3. Maj M: Clinical prediction of response to lithium prophylaxis in bipolar patients: the importance of the previous pattern of course of the illness. Clin Neuropharmacol 1990; 13(suppl 1):S66-S70Google Scholar
4. Maj M, Del Vecchio M, Starace F, Pirozzi R, Kemali D: Prediction of affective psychoses response to lithium prophylaxis: the role of sociodemographic, clinical, psychological and biological variables. Acta Psychiatr Scand 1984; 69:37–44Crossref, Medline, Google Scholar
5. Kleindienst N, Greil W: Lithium in the long-term treatment of bipolar disorders. Eur Arch Psychiatry Clin Neurosci 2003; 253:120–125Crossref, Medline, Google Scholar
6. Kleindienst N, Greil W: Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology 2000; 42(suppl 1):2-10Google Scholar
7. Grof P, Alda M, Grof E, Fox D, Cameron P: The challenge of predicting response to stabilising lithium treatment: the importance of patient selection. Br J Psychiatry Suppl 1993; 21:16–19Medline, Google Scholar
8. Goodwin FK, Jamison KR: Manic-Depressive Illness. New York, Oxford University Press, 1990Google Scholar
9. Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT: Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry 2000; 48:30–42Crossref, Medline, Google Scholar
10. Krüger S, Seminowicz D, Goldapple K, Kennedy SH, Mayberg HS: State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry 2003; 1274-1283Google Scholar
11. Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P: Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry 2002; 159:1830–1840Link, Google Scholar
12. Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC: Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 2004; 16:1746–1772Crossref, Medline, Google Scholar
13. Collins DL, Neelin P, Peters TM, Evans AC: Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 1994; 18:192–205Crossref, Medline, Google Scholar
14. Brett M, Johnsrude IS, Owen AM: The problem of functional localization in the human brain. Nat Rev Neurosci 2002; 3:243–249Crossref, Medline, Google Scholar
15. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675–682Abstract, Google Scholar
16. Angrilli A, Palomba D, Cantagallo A, Maietti A, Stegagno L: Emotional impairment after right orbitofrontal lesion in a patient without cognitive deficits. Neuroreport 1999; 10:1741–1746Crossref, Medline, Google Scholar
17. Grafman J, Vance SC, Weingartner H, Salazar AM, Amin D: The effects of lateralized frontal lesions on mood regulation. Brain 1986; 109:1127–1148Crossref, Medline, Google Scholar
18. Joseph R: Frontal lobe psychopathology: mania, depression, confabulation, catatonia, perseveration, obsessive compulsions, and schizophrenia. Psychiatry 1999; 62:138–172Crossref, Medline, Google Scholar
19. Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ: Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport 2000; 11:1739–1744Crossref, Medline, Google Scholar
20. Elliott R, Baker SC, Rogers RD, O’Leary DA, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ: Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med 1997; 27:931–942Crossref, Medline, Google Scholar
21. Bechara A, Damasio H, Damasio AR: Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 2000; 10:295–307Crossref, Medline, Google Scholar
22. Rolls E: A theory of emotion and its application to understanding the neural basis of emotion. Cognition and Emotion 1990; 4:161–190Crossref, Google Scholar
23. Rolls ET: The orbitofrontal cortex and reward. Cereb Cortex 2000; 10:284–294Crossref, Medline, Google Scholar
24. Schultz W: Reward signaling by dopamine neurons. Neuroscientist 2001; 7:293–302Crossref, Medline, Google Scholar
25. Schultz W: The reward signal of midbrain dopamine neurons. News Physiol Sci 1999; 14:249–255Medline, Google Scholar
26. Vogt BA, Pandya DN: Cingulate cortex of the rhesus monkey, II: cortical afferents. J Comp Neurol 1987; 262:271–289Crossref, Medline, Google Scholar
27. Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ: Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 1997; 154:926–933Link, Google Scholar
28. Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL: The Counting Stroop: an interference task specialized for functional neuroimaging—validation study with functional MRI. Hum Brain Mapp 1998; 6:270–282Crossref, Medline, Google Scholar
29. Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL: The Emotional Counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 1998; 44:1219–1228Crossref, Medline, Google Scholar
30. Keightley ML, Seminowicz DA, Bagby RM, Costa PT, Fossati P, Mayberg HS: Personality influences limbic-cortical interactions during sad mood induction. Neuroimage 2003; 20:2031–2039Crossref, Medline, Google Scholar
31. Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S: In search of the self: a PET investigation of self-referential information. Psychol Sci 1999; 10:26–34Crossref, Google Scholar
32. Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H: In search of the emotional self: an fMRI study using positive and negative emotional words. Am J Psychiatry 2003; 160:1938–1945Link, Google Scholar
33. Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD: Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 2002; 14:1215–1229Crossref, Medline, Google Scholar
34. Gusnard DA, Akbudak E, Shulman GL, Raichle ME: Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 2001; 98:4259–4264Crossref, Medline, Google Scholar
35. Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD: Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 1995; 152:1576–1585Link, Google Scholar
36. Maj M: The impact of lithium prophylaxis on the course of bipolar disorder: a review of the research evidence. Bipolar Disord 2000; 2:93–101Crossref, Medline, Google Scholar
37. Greil W, Kleindienst N, Erazo N, Muller-Oerlinghausen B: Differential response to lithium and carbamazepine in the prophylaxis of bipolar disorder. J Clin Psychopharmacol 1998; 18:455–460Crossref, Medline, Google Scholar
38. Colom F, Vieta E, Reinares M, Martinez-Aran A, Torrent C, Goikolea JM, Gasto C: Psychoeducation efficacy in bipolar disorders: beyond compliance enhancement. J Clin Psychiatry 2003; 64:1101–1105Crossref, Medline, Google Scholar
39. Colom F, Vieta E, Martinez-Aran A, Reinares M, Goikolea JM, Benabarre A, Torrent C, Comes M, Corbella B, Parramon G, Corominas J: A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry 2003; 60:402–407Crossref, Medline, Google Scholar
40. Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H: Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004:1:34-41Google Scholar
41. Maj M, Pirozzi R, Magliano L, Bartoli L: Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry 1998; 155:30–35Link, Google Scholar
42. Bauer M, Alda M, Priller J, Young LT: Implications of the neuroprotective effects of lithium for the treatment of bipolar and neurodegenerative disorders. Pharmacopsychiatry 2003; 36(suppl 3):S250-S254Google Scholar
43. Bowden CL: Predictors of response to divalproex and lithium. J Clin Psychiatry 1995; 56(suppl 3):25-30Google Scholar
44. Martinez-Aran A, Vieta E, Colom F, Torrent C, Sanchez-Moreno J, Reinares M, Benabarre A, Goikolea JM, Brugue E, Daban C, Salamero M: Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord 2004; 6:224–232Crossref, Medline, Google Scholar
45. Martinez-Aran A, Vieta E, Colom F, Reinares M, Benabarre A, Gasto C, Salamero M: Cognitive dysfunctions in bipolar disorder: evidence of neuropsychological disturbances. Psychother Psychosom 2000; 69:2–18Crossref, Medline, Google Scholar
46. Jackson DC, Malmstadt JR, Larson CL, Davidson RJ: Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology 2000; 37:515–522Crossref, Medline, Google Scholar
47. Baxter LRJ, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM: Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46:243–250Crossref, Medline, Google Scholar
48. Dolan RJ, Bench CJ, Brown RG, Scott LC, Friston KJ, Frackowiak RS: Regional cerebral blood flow abnormalities in depressed patients with cognitive impairment. J Neurol Neurosurg Psychiatry 1992; 55:768–773Crossref, Medline, Google Scholar
49. Meyer JH, Kapur S, Eisfeld B, Brown GM, Houle S, DaSilva J, Wilson AA, Rafi-Tari S, Mayberg HS, Kennedy SH: The effect of paroxetine on 5-HT2A receptors in depression: an [18F]setoperone PET imaging study. Am J Psychiatry 2001; 158:78–85Link, Google Scholar
50. Mayberg HS, Lewis PJ, Regenold W, Wagner HNJ: Paralimbic hypoperfusion in unipolar depression. J Nucl Med 1994; 35:929–934Medline, Google Scholar
51. Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C: Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry 1997; 41:15–22Crossref, Medline, Google Scholar
52. Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ: The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol Med 1992; 22:607–615Crossref, Medline, Google Scholar
53. Videbech P: PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand 2000; 101:11–20Crossref, Medline, Google Scholar
54. Phillips ML, Medford N, Young AW, Williams L, Williams SC, Bullmore ET, Gray JA, Brammer MJ: Time courses of left and right amygdalar responses to fearful facial expressions. Hum Brain Mapp 2001; 2:193–202Crossref, Google Scholar



