Clinical Correlates and Familial Aggregation of Age at Onset in Bipolar Disorder
Abstract
OBJECTIVE: To assess whether age at onset variation reflects underlying genetic heterogeneity in bipolar disorder, the authors examined the clinical and familial characteristics of age at onset in bipolar disorder subjects from families with multiple affected members. METHOD: A total of 211 families with 1,856 subjects were ascertained through bipolar I disorder probands. All the subjects were assessed with the Diagnostic Interview for Genetic Studies and assigned diagnoses by trained clinicians using best estimate procedures. Admixture analysis with the 211 bipolar disorder probands was used to decompose the age-at-onset distribution into a mixture of theoretical normal distributions. Logistic regression with general estimating equations was then used to examine clinical correlates and familial aggregation of age at onset in all 717 bipolar disorder subjects. RESULTS: The age-at-onset distribution consisted of a mixture of three normal distributions with means of 16.6 (SD=5.1), 26.0 (SD=1.4), and 34.7 (SD=6.6) years that comprised 79.7%, 7.2%, and 13.1% of the group, respectively. Cutoff points at ages 21 and 28 were derived from this analysis and used to define age-at-onset subgroups. Early-onset (age at onset ≤21) subjects had higher risks of drug abuse, alcohol abuse, rapid cycling, and suicide attempts. Affected subjects from a family with an early-onset proband were more likely than others to have an early onset (odds ratio=4.53, 95% CI=3.09–6.64). Subjects from a family with a proband whose age at onset was <28 were also more likely to have higher risks of drug abuse (odds ratio=11.62, 95% CI=2.16–62.66). CONCLUSIONS: Age at onset is associated with clinical heterogeneity in bipolar disorder and aggregates, possibly along with drug abuse, within families. These findings are consistent with the conclusion that age at onset reflects underlying genetic heterogeneity in bipolar disorder. Thus, age at onset may conceivably be used to identify more homogeneous groups of bipolar disorder families and thereby facilitate the mapping of bipolar disorder susceptibility genes.
It has been shown that variation in age at onset reflects the underlying genetic heterogeneity of several complex diseases such as Alzheimer’s disease (1–4) and breast cancer (5, 6). By using early age at onset to identify more homogeneous subgroups, investigators have been able to identify several genes that contribute to the etiology of these diseases.
Age at onset variation may similarly reflect underlying genetic heterogeneity in bipolar disorder. Recent studies have suggested that there may be a mixture of overlapping distributions of age at onset in bipolar disorder (7, 8), while other studies have shown that the clinical presentation of bipolar disorder, such as the occurrence of comorbid psychiatric disorders, may vary considerably with age at onset (9–12). In addition, some studies have reported that age at onset aggregates within bipolar disorder families (13, 14), and a segregation analysis has found that the transmission of bipolar disorder may differ in early- versus late-onset bipolar disorder (15).
We sought to extend these findings by using data from the NIMH Genetics Initiative for Bipolar Disorder to answer the following questions: 1) Can we replicate the finding that there is a mixture of overlapping age-at-onset distributions that define subgroups of bipolar disorder? 2) Does the clinical presentation of bipolar disorder differ across these age-at-onset subgroups? 3) Do the age-at-onset subgroups aggregate within families? 4) Does age at onset co-aggregate in families with other clinical features? Our goal was to assess the clinical evidence that variation in age at onset along with other clinical features reflects the underlying genetic heterogeneity of bipolar disorder and may be used to identify more homogeneous subgroups of families to facilitate mapping susceptibility genes that contribute to this disorder.
Method
Subjects
The subjects included in this study came from families collected as part of the NIMH Genetics Initiative for Bipolar Disorder. Details of the ascertainment and assessment of these subjects have been described in detail elsewhere (16). Briefly, a total of 211 families (1,856 subjects) were ascertained at one of four collaborating sites (Indiana University, Washington University, Johns Hopkins University, and the NIMH Intramural Research Program) using standardized criteria. Each family had to have a proband with bipolar I disorder and at least one first-degree relative affected with either bipolar I disorder or schizoaffective disorder–bipolar type. Subjects were assessed by trained mental health professionals using the Diagnostic Interview for Genetic Studies. Diagnoses were then assigned by psychiatrists on the basis of all available information—which included the Diagnostic Interview for Genetic Studies data, family history data, and medical records—using best estimate procedures with DSM-III-R criteria (for bipolar I disorder or schizoaffective disorder–bipolar type) and Research Diagnostic Criteria (for bipolar II disorder or recurrent unipolar depression). We examined a narrow phenotype model of affection status that included bipolar I disorder, schizoaffective disorder–bipolar type, and bipolar II disorder. We also analyzed a broad phenotype model that further included recurrent unipolar depression, but since the results were very similar we report here only the results with the narrow model. Age at onset was defined on the basis of self-report as the age at first episode of mania or depression that met diagnostic criteria. The Diagnostic Interview for Genetic Studies also provided detailed information about a range of auxiliary clinical features and comorbid psychiatric disorders diagnosed according to DSM-III-R criteria.
Statistical Analyses
We used analysis of variance to examine if age at onset differed by four age-at-contact groups and three phenotype subgroups and used a chi-square test to examine if age at onset differed by gender. Because the age-at-onset distribution of our sample was not normal, we logarithmically transformed the data for this descriptive analysis.
To determine whether the observed age-at-onset distribution could be decomposed into a mixture of theoretical normal distributions, we used admixture analysis with maximum likelihood estimation of the finite normal mixture (17). We tested a series of mixture models with the assumption of an increasing number of theoretical component distributions and used Akaike’s information criterion to select the model that best fit the observed distribution most parsimoniously. We used only the 211 bipolar I disorder probands for this analysis, since the method cannot take into account intrafamilial correlation present in the full dataset of related subjects. Ignoring the intrafamilial correlation may bias the estimates of the standard deviations of the theoretical age-at-onset distributions.
We used the results of the admixture analysis to derive age-at-onset cutoff points for dividing the affected subjects into subgroups. This was done by adding or subtracting one standard deviation from the estimated means of the theoretical component distributions. We then compared the prevalence rates of clinical correlates across the resulting subgroups. Clinical correlates of interest included comorbid disorders (such as drug abuse, alcohol abuse, panic disorder, obsessive-compulsive disorder, and eating disorders) and auxiliary clinical features (such as rapid cycling, suicide attempt, mood-congruent psychotic symptoms, and episode frequency). Differences in the rates of these features across age-at-onset subgroups were first examined by using chi-square tests and then with logistic regression that controlled for age at contact and gender. We controlled for age at contact and gender because both were associated with age at onset (Table 1) and possibly with the clinical features of interest. Therefore, both were potential confounders of the relationships we sought to examine and required adjustment. By controlling for age at contact, in particular, we tried to account for the fact that the manifestation of the clinical features we examined were potentially age dependent and the subjects were interviewed at different ages (i.e., observations on them were “censored”). Logistic regression models were fit using general estimating equations to account for the potential residual correlation in outcomes among affected subjects from the same families.
We then examined the familial aggregation of age at onset using a proband-predictive model with logistic regression. Here, we modeled dichotomous age at onset (≤21 versus >21) in siblings as a function of age at onset in the proband, controlling for continuous age at contact and gender of the siblings. We modeled age at onset in probands both as a dichotomous variable (≤21 versus >21) and as a dummy-coded categorical variable with three groups (≤21, 22–28, and >28 as the reference). General estimating equations were again used to account for residual correlation among siblings.
We used a modification of the proband-predictive model (18) to investigate potential co-aggregation of different clinical correlates with age at onset in these bipolar disorder families. Here, co-aggregation was assessed by examining whether age at onset in the proband predicted the clinical correlate of interest in the siblings after we controlled for the clinical correlate in the proband as well as age at onset, age at contact, and gender of the relative. These models were also fit by using logistic regression with general estimating equations. All proband-predictive models tested controlled for age at contact and sex for the aforementioned reasons.
All analyses were carried out by using the software Stata, release 8 (College Station, Tex., Stata Corp.). Results from logistic regression models are reported as odds ratios with 95% confidence intervals (CIs). The reported p values were not corrected for multiple testing. Many of the clinical features we examined were selected because prior considerations suggested these features may be relevant to age at onset, and we prefer to rely on replication of findings in independent samples rather than use corrections that may be overly conservative.
Results
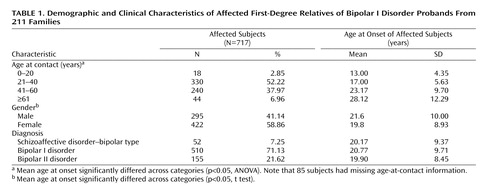
Table 1 shows the characteristics of the 717 subjects diagnosed with bipolar disorder in this study. The mean age at onset was significantly younger for female relative to male subjects, and the mean age at onset increased in relation to the age of contact. The mean ages at onset for subjects with schizoaffective disorder–bipolar type, bipolar I disorder, and bipolar II disorder were very similar.
The distribution of age at onset in the probands from the 211 families was skewed. Admixture analysis yielded a best fitting model (based on Akaike’s information criterion) that suggested the observed distribution could be decomposed into a mixture of three normal distributions (Figure 1). The three component age-at-onset distributions had means of 16.6 (SD=5.1), 26.0 (SD=1.4), and 34.7 (SD=6.6) years and comprised 79.7%, 7.2%, and 13.1% of the group, respectively. On the basis of these results, we divided the group into three age-at-onset subgroups with cutoff points at ages 21 (mean of the youngest group plus one standard deviation) and 28 (mean of the oldest group minus one standard deviation). Alternatively, since the youngest group occupied a substantially greater proportion than the sum of the other two groups, we combined the second and third age-at-onset subgroups to dichotomize early- and late-onset subjects at a cutoff point of age 21.
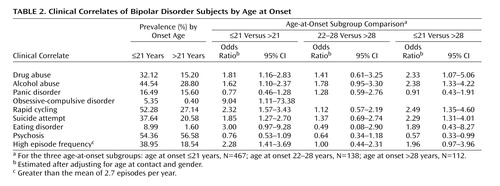
Table 2 shows the prevalence rates of each clinical correlate among the affected subjects by age at onset. Relative to subjects with a late onset, those with early-onset bipolar disorder were found to have significantly higher rates of drug abuse (z=4.73, p<0.001), alcohol abuse (z=4.08, p<0.001), obsessive-compulsive disorder (z=2.59, p<0.001), rapid cycling (z=5.81, p<0.001), suicide attempt (z=4.40, p<0.001), and episode frequency (z=3.90, p<0.001), with a higher rate of eating disorders that was nearly significant. Typically, the onset of the comorbid disorders occurred after the onset of bipolar disorder. For example, in over two-thirds of the cases the onset of drug or alcohol abuse was after the onset of bipolar disorder. Logistic regression analyses confirmed that early-onset subjects were at a higher risk of having these clinical correlates even after gender and age at contact were controlled. In general, subjects with an age at onset between 22 and 28 had similar, albeit slightly higher, risks relative to subjects with an age at onset >28. There were no apparent differences among the age-at-onset subgroups in the rates of panic disorder or mood-congruent psychosis. Conversely, early-onset subjects tended to have lower rates of psychosis, especially when comparing those with an onset of bipolar disorder ≤21 versus >28.
We used proband-predictive logistic regression models to examine whether age at onset aggregated in these families. Specifically, we tested if age at onset in the probands predicted age at onset in the siblings after age at contact and gender were controlled (Table 3). Affected siblings of early-onset probands were over four times more likely than others to have an early onset. When we trichotomized age at onset in the probands (model 2), we found that compared with siblings of probands in the oldest age-at-onset group (>28), those in the youngest age-at-onset group (≤21) were significantly more likely to have an early onset. By contrast, siblings of probands in the middle age-at-onset group were no more or less likely to have an early onset.
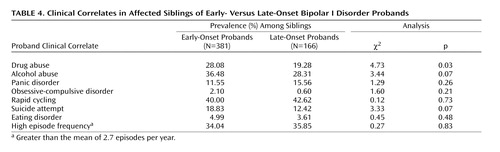
We then compared the prevalence rates of different clinical features among siblings of early- versus late-onset probands (Table 4). The rates of suicide attempts and alcohol abuse were marginally higher in siblings of early-onset probands. However, the most compelling difference was observed for drug abuse. Prevalence rates of the other clinical features did not differ significantly among siblings of early- versus late-onset probands.
On the basis of these initial comparisons, we used a modification of the proband-predictive logistic regression model to more closely examine the familial co-aggregation of age at onset and drug abuse. Specifically, we tested whether age at onset in the probands predicted drug abuse in the siblings after we controlled for drug abuse in the probands as well as age at onset, age at contact, and gender of the siblings (Table 5). In model 1 with dichotomous onset classification, proband age at onset did not appear to predict drug abuse in the siblings (odds ratio=0.96, 95% CI=0.44–2.09). However, in model 2 which had a trichotomous classification of proband age at onset, a significant association with drug abuse in the siblings was revealed. Compared with siblings of probands in the oldest age-at-onset group, those in the youngest age-at-onset group were over 10 times more likely to have drug abuse, and those in the middle age-at-onset group were over 18 times more likely. Because of the similar increase in risk, we collapsed the middle and youngest age-at-onset categories and found that siblings of probands with age at onset ≤28 were over 11 times more likely to have drug abuse (odds ratio=11.62, 95% CI=2.16–62.56) than siblings of probands with age at onset >28.
We reran the aforementioned models and controlled for disease duration (age at contact minus age at onset) instead of age at contact. Like age at contact, disease duration was correlated with age at onset (Pearson correlation r=–0.16, p<0.001) and may have also been an important potential confounder of the relationships we observed. However, the results with disease duration controlled were not meaningfully different (data not shown).
Discussion
We found that the skewed distribution of age at onset in our bipolar I disorder subjects could be decomposed by admixture analysis into three normal distributions with means of 16.6 (SD=5.1), 26.0 (SD=1.34), and 34.7 (SD=6.6) years that comprised 79.7%, 7.2%, and 13.1% of the group, respectively. Although there were differences in the estimated means and proportions of the component distributions, our findings were largely consistent with those reported by Bellivier et al. (7, 8) in their studies of age at onset with two independent samples.
We used the results from our admixture analysis to empirically define cutoff points at ages 21 and 28 for distinguishing age-at-onset subgroups. We then examined if the clinical presentations of bipolar disorder differed across the age-at-onset subgroups. Consistent with previous studies, we found that early-onset subjects (age at onset ≤21) had higher risks of clinical features such as comorbid substance abuse (19, 20), suicidality (11, 20–23), rapid cycling (11, 24), and increased episode frequency (25). We also found that early-onset subjects were at greater risk for eating disorders (although this difference did not quite reach significance after gender and age at contact were controlled), an association that to our knowledge has not been previously examined. In general, subjects with an age at onset between 22 and 28 years were relatively similar in presentation to late-onset subjects (age at onset >28), albeit with slightly higher risks of the various features examined. These findings appear to suggest that early-onset bipolar disorder has a more severe course leading to the manifestation of numerous clinical complications. However, in contrast to previous studies, we did not find any association between early-onset bipolar disorder and higher risks of comorbid panic disorder (10, 26). Furthermore, we found that early-onset bipolar disorder tended to be associated with lower rates of mood-congruent psychotic symptoms. Such findings contradict some earlier studies of mood-congruent psychosis (27).
As noted by Bellivier and colleagues (7), “if age at onset is a marker for biologically different subtypes of bipolar disorder, then age-at-onset subgroups should have separate normal distributions with different means, variances, and population proportions as well as different clinical characteristics.” Our results, therefore, would appear to support the conclusion that age at onset is a clinical marker of biological heterogeneity in bipolar disorder.
In order to assess whether the suggested biological heterogeneity of bipolar disorder may be due to genetic heterogeneity, we examined whether the age-at-onset subgroups aggregated in these bipolar disorder families. Using a proband predictive model, we showed that affected siblings of early-onset probands were over four times more likely than others to have an early onset as well, even after we controlled for age at contact, which was highly correlated with the self-reported age at onset in this sample. Several previous studies have reported similar observations, which suggests that age at onset aggregates in families. Such findings are consistent with the notion that age at onset is a heritable trait within bipolar disorder reflecting the underlying genetic heterogeneity of the disorder. However, it should be noted that the findings do not prove this, since familial aggregation may be due to nongenetic as well as genetic factors.
We further noted that siblings of early-onset probands tended to have higher risks of certain psychiatric disturbances, including alcoholism, drug abuse, and suicidality. It is interesting that several previous studies also found that these same disturbances occurred more frequently in relatives of early-onset subjects (19, 28–30). Because our results were most compelling with drug abuse, we sought to examine more closely the evidence that it co-aggregates with age at onset in our families. Using a modification of the proband-predictive model, we found that siblings of probands with an age at onset <21 were over 10 times more likely to have drug abuse problems than siblings of probands with an age at onset >28. This association persisted even after we controlled for age at onset in the siblings and drug abuse in the probands, which could confound the relationship. In contrast to our other findings, the siblings of probands with ages at onset between 22–28 were more similar to the early-onset than the late-onset groups. It is unclear why this is the case. Nevertheless, the findings suggest age at onset and drug abuse may share a common genetic etiology that warrants further investigation.
The current study has several limitations. Most notably, age at onset was assessed by retrospective self-report. The most accurate way to assess age at onset would be to prospectively follow normal subjects until they develop bipolar disorder. However, this is impractical in most situations. By default, the next best approach is self-report. The quality of such data is potentially degraded by recall or information bias. Indeed, it is arguable whether subjects can accurately tell whether their disorder started when they were 16 or 18 years old. Consequently, the true variation in age at onset of bipolar disorder may not be validly captured by a self-reported continuous variable. It seems more reasonable to assume that subjects are better able to distinguish whether their disorder started when they were 16 versus 30 years old. In this case, capturing age at onset in broad categories may be the best that can be validly differentiated.
The difficulty with a categorical variable is determining where to make the cutoff points between age-at-onset subgroups. Previous studies have used a variety of cutoff points (10, 15, 31), often with little justification. We decided to empirically define the cutoff points using the “points of rarity” in the observed age-at-onset distribution. Such an approach has a certain intuitive appeal, but it too may lead to misclassification of subjects, especially among those who have an age at onset where the subgroups may overlap. Misclassification of this sort would tend to introduce noise into the analyses and likely bias observed associations toward the null. Despite this, we were still able to observe robust patterns of differences between the age-at-onset subgroups.
The current study also has several important strengths. The families used in this study were ascertained through a common set of criteria, and the subjects were all assessed with the Diagnostic Interview for Genetic Studies, a widely used and well-validated instrument that collects information on a wide range of psychopathology. Furthermore, diagnoses of mood disorders and other comorbid conditions were assigned by experienced clinicians using best estimate procedures, thus minimizing concerns about misclassification of affection status. Finally, the study group was sufficiently large to allow examination of a variety of hypotheses related to the clinical and familial features of age at onset in bipolar disorder.
In summary, we found that 1) the distribution of age at onset in bipolar disorder revealed several subgroups of patients, 2) the clinical presentation varied significantly across these subgroups, and 3) the subgroups aggregated within families. These results, which are consistent with several other lines of evidence, provide further support for the conclusion that variation in age at onset is an important clinical marker of the underlying genetic heterogeneity in bipolar disorder. Thus, age at onset may conceivably be used to identify more homogeneous groups of bipolar disorder families and thereby facilitate the mapping of bipolar disorder susceptibility genes. Some investigators have started to consider age at onset in genetic studies of bipolar disorder (32–34), but the current findings suggest further investigation is warranted. Finally, our novel finding that drug abuse may co-aggregate with early age at onset requires further investigation.
 |
 |
 |
 |
 |
Received Sept. 17, 2004; revision received Jan. 12, 2005; accepted March 18, 2005. From the Department of Mental Health (Bloomberg School of Public Health) and Department of Psychiatry and Behavioral Sciences (School of Medicine), Johns Hopkins University, Baltimore. Address correspondence and reprint requests to Dr. Zandi, 624 N. Broadway, 8th Floor, Baltimore, MD 21205; [email protected] (e-mail).Additional information on this study accompanies the online version of the article.
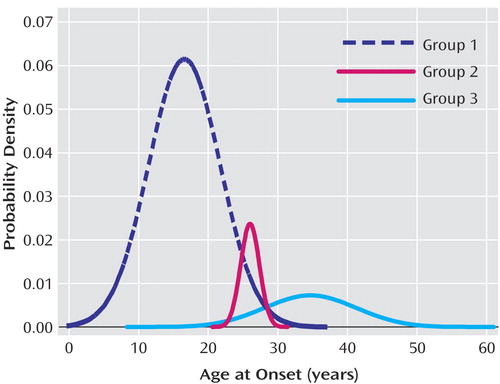
Figure 1. Three Theoretical Distributions of Age at Illness Onset for Bipolar I Disorder Probands From 211 Families
1. Pericak-Vance MA, Bebout JL, Gaskell PC Jr, Yamaoka LH, Hung WY, Alberts MJ, Walker AP, Bartlett RJ, Haynes CA, Welsh KA, et al: Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet 1991; 48:1034–1050Medline, Google Scholar
2. Brooks JO III: A comment on age at onset as a subtype of Alzheimer disease. Alzheimer Dis Assoc Disord 1995; 9(suppl 1):S28-S29Google Scholar
3. Scott WK, Grubber JM, Conneally PM, Small GW, Hulette CM, Rosenberg CK, Saunders AM, Roses AD, Haines JL, Pericak-Vance MA: Fine mapping of the chromosome 12 late-onset Alzheimer disease locus: potential genetic and phenotypic heterogeneity. Am J Hum Genet 2000; 66:922–932Crossref, Medline, Google Scholar
4. Macciardi F, Cavallini MC: A two-locus model for familial Alzheimer’s disease? Genet Epidemiol 1993; 10:437–441Crossref, Medline, Google Scholar
5. Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, King MC: Linkage of early-onset familial breast cancer to chromosome 17q21. Science 1990; 250:1684–1689Crossref, Medline, Google Scholar
6. King MC: Localization of the early-onset breast cancer gene. Hosp Pract (Off Ed) 1991; 26:121–126Medline, Google Scholar
7. Bellivier F, Golmard JL, Henry C, Leboyer M, Schurhoff F: Admixture analysis of age at onset in bipolar I affective disorder. Arch Gen Psychiatry 2001; 58:510–512Crossref, Medline, Google Scholar
8. Bellivier F, Golmard J-L, Rietschel M, Schulze TG, Malafosse A, Preisig M, McKeon P, Mynett-Johnson L, Henry C, Leboyer M: Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry 2003; 160:999–1001Link, Google Scholar
9. McElroy SL, Strakowski SM, West SA, Keck PE Jr, McConville BJ: Phenomenology of adolescent and adult mania in hospitalized patients with bipolar disorder. Am J Psychiatry 1997; 154:44–49Link, Google Scholar
10. Schurhoff F, Bellivier F, Jouvent R, Mouren-Simeoni MC, Bouvard M, Allilaire JF, Leboyer M: Early and late onset bipolar disorders: two different forms of manic-depressive illness? J Affect Disord 2000; 58:215–221Crossref, Medline, Google Scholar
11. Carter TD, Mundo E, Parikh SV, Kennedy JL: Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res 2003; 37:297–303Crossref, Medline, Google Scholar
12. McGlashan TH: Adolescent versus adult onset of mania. Am J Psychiatry 1988; 145:221–223Link, Google Scholar
13. Leboyer M, Bellivier F, McKeon P, Albus M, Borrman M, Perez-Diaz F, Mynett-Johnson L, Feingold J, Maier W: Age at onset and gender resemblance in bipolar siblings. Psychiatry Res 1998; 81:125–131Crossref, Medline, Google Scholar
14. O’Mahony E, Corvin A, O’Connell R, Comerford C, Larsen B, Jones R, McCandless F, Kirov G, Cardno AG, Craddock N, Gill M: Sibling pairs with affective disorders: resemblance of demographic and clinical features. Psychol Med 2002; 32:55–61Crossref, Medline, Google Scholar
15. Grigoroiu-Serbanescu M, Martinez M, Nothen MM, Grinberg M, Sima D, Propping P, Marinescu E, Hrestic M: Different familial transmission patterns in bipolar I disorder with onset before and after age 25. Am J Med Genet 2001; 105:765–773Crossref, Medline, Google Scholar
16. McInnis MG, Lan TH, Willour VL, McMahon FJ, Simpson SG, Addington AM, MacKinnon DF, Potash JB, Mahoney AT, Chellis J, Huo Y, Swift-Scanlan T, Chen H, Koskela R, Stine OC, Jamison KR, Holmans P, Folstein SE, Ranade K, Friddle C, Botstein D, Marr T, Beaty TH, Zandi P, DePaulo JR: Genome-wide scan of bipolar disorder in 65 pedigrees: supportive evidence for linkage at 8q24, 18q22, 4q32, 2p12, and 13q12. Mol Psychiatry 2003; 8:288–298Crossref, Medline, Google Scholar
17. Kolenikov S: DENORMIX: Stata module to perform decomposition of normal mixture. http://econpapers.repec.org/software/bocbocode/s416605.htmGoogle Scholar
18. Hudson JI, Laird NM, Betensky RA: Multivariate logistic regression for familial aggregation of two disorders, I: development of models and methods. Am J Epidemiol 2001; 153:500–505Crossref, Medline, Google Scholar
19. Bashir M, Russell J, Johnson G: Bipolar affective disorder in adolescence: a 10-year study. Aust NZ J Psychiatry 1987; 21:36–43Medline, Google Scholar
20. Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, Bowden CL, Sachs GS, Nierenberg AA: Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 2004; 55:875–881Crossref, Medline, Google Scholar
21. Lopez P, Mosquera F, de Leon J, Gutierrez M, Ezcurra J, Ramirez F, Gonzalez-Pinto A: Suicide attempts in bipolar patients. J Clin Psychiatry 2001; 62:963–966Crossref, Medline, Google Scholar
22. Tsai SY, Lee JC, Chen CC: Characteristics and psychosocial problems of patients with bipolar disorder at high risk for suicide attempt. J Affect Disord 1999; 52:145–152Crossref, Medline, Google Scholar
23. Engstrom C, Brandstrom S, Sigvardsson S, Cloninger R, Nylander PO: Bipolar disorder, II: personality and age of onset. Bipolar Disord 2003; 5:340–348Crossref, Medline, Google Scholar
24. Yildiz A, Sachs GS: Characteristics of rapid cycling bipolar-I patients in a bipolar speciality clinic. J Affect Disord 2004; 79:247–251Crossref, Medline, Google Scholar
25. Almeida OP, Fenner S: Bipolar disorder: similarities and differences between patients with illness onset before and after 65 years of age. Int Psychogeriatr 2002; 14:311–322Crossref, Medline, Google Scholar
26. Henry C, Van den Bulke D, Bellivier F, Etain B, Rouillon F, Leboyer M: Anxiety disorders in 318 bipolar patients: prevalence and impact on illness severity and response to mood stabilizer. J Clin Psychiatry 2003; 64:331–335Crossref, Medline, Google Scholar
27. Rosen LN, Rosenthal NE, Van Dusen PH, Dunner DL, Fieve RR: Age at onset and number of psychotic symptoms in bipolar I and schizoaffective disorder. Am J Psychiatry 1983; 140:1523–1524Link, Google Scholar
28. Somanath CP, Jain S, Reddy YC: A family study of early-onset bipolar I disorder. J Affect Disord 2002; 70:91–94Crossref, Medline, Google Scholar
29. Strober M, Morrell W, Burroughs J, Lampert C, Danforth H, Freeman R: A family study of bipolar I disorder in adolescence: early onset of symptoms linked to increased familial loading and lithium resistance. J Affect Disord 1988; 15:255–268Crossref, Medline, Google Scholar
30. Weissman MM, Gershon ES, Kidd KK, Prusoff BA, Leckman JF, Dibble E, Hamovit J, Thompson WD, Pauls DL, Guroff JJ: Psychiatric disorders in the relatives of probands with affective disorders: the Yale University-National Institute of Mental Health Collaborative Study. Arch Gen Psychiatry 1984; 41:13–21Crossref, Medline, Google Scholar
31. Carlson GA, Bromet EJ, Driessens C, Mojtabai R, Schwartz JE: Age at onset, childhood psychopathology, and 2-year outcome in psychotic bipolar disorder. Am J Psychiatry 2002; 159:307–309Link, Google Scholar
32. Ospina-Duque J, Duque C, Carvajal-Carmona L, Ortiz-Barrientos D, Soto I, Pineda N, Cuartas M, Calle J, Lopez C, Ochoa L, Garcia J, Gomez J, Agudelo A, Lozano M, Montoya G, Ospina A, Lopez M, Gallo A, Miranda A, Serna L, Montoya P, Palacio C, Bedoya G, McCarthy M, Reus V, Freimer N, Ruiz-Linares A: An association study of bipolar mood disorder (type I) with the 5-HTTLPR serotonin transporter polymorphism in a human population isolate from Colombia. Neurosci Lett 2000; 292:199–202Crossref, Medline, Google Scholar
33. Papadimitriou GN, Dikeos DG, Karadima G, Avramopoulos D, Daskalopoulou EG, Vassilopoulos D, Stefanis CN: Association between the GABA(A) receptor alpha5 subunit gene locus (GABRA5) and bipolar affective disorder. Am J Med Genet 1998; 81:73–80Crossref, Medline, Google Scholar
34. Benedetti F, Bernasconi A, Lorenzi C, Pontiggia A, Serretti A, Colombo C, Smeraldi E: A single nucleotide polymorphism in glycogen synthase kinase 3-beta promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci Lett 2004; 355:37–40Crossref, Medline, Google Scholar



