New-Onset Bipolar Disorder in Late Life: A Case of Mistaken Identity
A 60-year-old, right-handed, previously successful and psychiatrically healthy businessman was brought by his family to a university hospital neuropsychiatry service for consultation regarding behavioral and personality changes. When he was age 55 years, his customarily excellent financial and social judgment began to decline. Over the next 4 years, his attention to personal hygiene deteriorated, his business decisions became financially and ethically unsound, his range of social interests narrowed dramatically, and he developed an insatiable “sweet tooth.” In the year preceding the consultation, his ability to maintain sleep diminished, he began spending money recklessly and impulsively and became unable to appreciate the feelings and concerns of others, and his speech and behavior took on a perseverative quality. Concurrently, he developed unprovoked, brief, frequent, and excessively intense episodes of tearfulness and laughing. These episodes lasted minutes at most, after which he would return to his usual euthymic emotional state. One month before the neuropsychiatric consultation, he had received a diagnosis of late-onset bipolar disorder and had begun treatment with lithium carbonate. When his serum lithium level reached the therapeutic range, his cognitive, behavioral, and motor function declined precipitously, prompting the consultation for a second diagnostic opinion. Is this patient’s presentation consistent with late-onset bipolar disorder? What assessments are needed to clarify his diagnosis?
The Clinical Problem
Bipolar Disorder in Late Life
Persons age 60 years and older may constitute as much as 25% of the population with bipolar disorder (1). However, the frequency of new-onset type I or type II bipolar disorder declines with advanced age (2, 3), with as few as 6%–8% of all new cases of bipolar disorder developing in persons age 60 years and older (1, 4). Bipolar disorder that develops in late life differs little from earlier-onset bipolar disorder in most clinical and demographic features (4, 5). Among the most important differences between earlier- and late-onset bipolar I disorder are higher levels of premorbid psychosocial functioning (4, 6), less severe psychopathology (5), and a higher frequency of neurological (“organic”) etiologies (2, 4) in the late-onset group. Among individuals with late-onset bipolar II disorder, atypical features, including “mood reactivity,” increased appetite or weight gain, hypersomnia, leaden paralysis, and/or a long-standing pattern of extreme sensitivity to perceived interpersonal rejection, are less common than among persons with earlier-onset forms of this condition (3). In a critical review of the literature on bipolar disorder in older adults, Depp and Jeste (2) found only weak or inconsistent evidence of a reduced frequency of a family history of bipolar disorder, an increased frequency of mixed episodes, and a less robust response to standard treatments among older persons with this condition. Chen et al. (7) reported that persons age 55 years or older with recent manic or mixed mood episodes respond well to treatment with either lithium or valproate when therapeutic serum levels are achieved. These observations suggest that the clinical presentation of persons with late-onset bipolar disorder is expected to conform to standard DSM diagnostic criteria for bipolar disorder and that these patients’ response to treatment is similar to that of younger persons with this disorder. When either or both of these expectations are not met, the diagnosis of late-onset bipolar disorder should be reconsidered.
Characterizing Emotional Disturbances: Mood Versus Affect
Central to the DSM-based diagnosis of late-onset bipolar disorder is a pervasive change in mood to a depressed, manic, or mixed state that is sustained over days to weeks. To make this diagnosis, the clinician must thoroughly evaluate the character and functional significance of the emotional disturbance and must also, and perhaps most importantly, carefully consider the temporal qualities of the disturbance.
In DSM-III-R and subsequent DSM editions, mood is defined primarily on temporal grounds as an emotional state sustained over a relatively long period of time (i.e., days to weeks). A metaphor is offered in which mood is described as the emotional “climate.” By contrast, affect is an observable pattern of behaviors that reflects an internal emotional state of comparatively short duration (i.e., minutes to hours) superimposed on the prevailing mood. In the same metaphor, affect is the emotional “weather.”
With mood and affect defined temporally, their subjective (experienced) and objective (expressed) components can then be characterized (8, 9). In general, the subjective and objective aspects of both mood and affect are congruent. For example, the depressed person feels and looks sad most of the day nearly every day for weeks; although the person may experience moments of relative happiness and smile, he or she returns quickly to feeling and appearing persistently sad when those moments pass. Less often, the subjective and objective aspects of either mood or affect may be incongruent. For example, geriatric patients with depression sometimes appear persistently sad but deny feeling so (incongruence between the subjective and objective components of mood). Clinicians also will encounter patients with paroxysms of crying who feel no sadness during those episodes (incongruence between the subjective and objective components of affect).
The clinical vignette illustrates the latter of these circumstances: a disorder of affect rather than a late-life disturbance of mood. The patient reported that during his episodes of crying or laughing, his feelings did not reflect his expression. In fact, he rejected the description of these episodes as crying or laughing and instead referred to them as episodes of “moisture,” because tearing occurred during both types of episode. Irrespective of his other neuropsychiatric symptoms and signs, this patient’s clinical presentation is not consistent with late-onset bipolar disorder because a distinct period of abnormally and persistently altered mood—the cardinal feature of this condition—was absent. The emotional disturbances with which he presented are nonetheless diagnostically informative: they are typical of pathological laughing and crying.
Pathological Laughing and Crying
Initially described by Wilson (10), pathological laughing and crying is a condition in which stereotyped affective displays occur without voluntary control or modulation, are not meaningfully related to the stimulus that provokes them, and do not produce a change in the prevailing mood (11, 12). These paroxysms of affective expression often occur without an understandable or predictable precipitant. For example, both sentimental and trivial stimuli may prompt intense crying or laughing. Occasionally, crying develops when laughing would be more contextually appropriate, or vice versa, and an episode may involve transition from laughing to crying, or the reverse, within a matter of seconds after onset. Patients with pathological laughing and crying may cry without feeling sad and laugh without feeling mirth or amusement, and they may do either or both regardless of their underlying mood and without producing a persistent disturbance of mood. Inferring from these episodes a subjective emotional state of any kind is inadvisable.
This condition produces added morbidity and suffering for patients and their families. The paroxysms of affect may be so frequent as to impair effective participation in rehabilitative and other therapies, may interfere with feeding or other basic care needs, and may be a source of considerable embarrassment and social disability for both patients and their families (8, 9).
Although Wilson (10) hypothesized that pathological laughing and crying develops as a result of impaired descending inhibition of brainstem emotional motor systems, more recent formulations suggest that this condition results from either anatomic or modulatory neurochemical dysfunction at critical nodes within a frontal-subcortical-thalamic-pontine-cerebellar network responsible for emotional regulation (9, 13). Pathological laughing and crying occurs commonly among patients with neurological conditions that disrupt the structure or function of this network, including stroke, amyotrophic lateral sclerosis, Parkinson’s disease, multiple sclerosis, frontotemporal dementia, traumatic brain injury, Alzheimer’s disease, epilepsy, normal pressure hydrocephalus, progressive supranuclear palsy, Wilson’s disease, and neurosyphilis, among others (8, 9). By contrast, pathological laughing and crying has not been described as a feature of primary psychiatric disorders in patients of any age. A diagnosis of pathological laughing and crying should in all cases prompt a thorough neuropsychiatric evaluation to assess this condition and any other neuropsychiatric symptoms and to identify the underlying neurological disorder responsible for the symptoms.
Neuropsychiatric Evaluation
Neuropsychiatric History and Interview
The medical history is the cornerstone of the neuropsychiatric evaluation. A comprehensive neuropsychiatric history includes description of developmental, medical, neurological, psychiatric, medication, substance-related, familial (genetic), and psychosocial influences on the major neuropsychiatric domains of cognition, emotion, behavior, and sensorimotor (elementary neurological) function. Particular attention is paid to symptoms that suggest a departure from age-related performance expectations, as well as to the pattern of changes across neuropsychiatric domains. In the clinical interview, the clinician seeks information with which to determine whether the presenting problems are new or old, episodic or persistent, static or progressive, and functionally important. Valid and reliable symptom inventories such as the Neuropsychiatric Inventory (14) and the Schedules for Clinical Assessment in Neuropsychiatry (15) are used routinely to define the presenting features more clearly and refine the differential diagnosis (16).
Neuropsychiatric Examination
The neuropsychiatric examination mirrors the clinical interview and entails a broader physical, neurological, and mental status examination than is often undertaken in general psychiatric settings. The physical examination focuses on the identification of signs of systemic illnesses with potential neuropsychiatric consequences (e.g., alcoholism, hypothyroidism, diabetes, vascular disease). The neurological examination includes evaluation of elementary neurological functions and also investigation for evidence of subtle (or so-called “soft”) signs, such as paratonia, primitive reflexes, and disturbances in higher cortical sensorimotor processing. The presence of one “less abnormal” primitive reflex (e.g., a glabellar, palmomental, or snout response) is generally not clinically important, especially if it is not sustained and not prominent in the examination. However, asymmetrical primitive reflexes or abnormal primitive reflexes, such as a suck, grasp, nuchocephalic, avoidance, or self-grasp response, and/or the presence of multiple (three or more) reflexes, particularly in the context of paratonia and/or other subtle neurological signs, are indicative of significant cerebral dysfunction (17–21).
The mental status examination is expanded to include not only screening assessments of memory and general cognitive functions such as the Mini-Mental State Examination (MMSE) (22) but also assessments of frontally mediated cognitive functions and time-sensitive cognitive performance such as the Frontal Assessment Battery (23) and Behavioral Dyscontrol Scale (24). Interpretation of performance on these measures is made by comparison to normative data (25) rather than raw (“cutoff”) scores in order to facilitate the distinction between normal age-related cognitive changes and overt cognitive impairment. When “bedside” assessments do not provide sufficient data to suggest a diagnosis, formal neuropsychological testing is undertaken.
Laboratory Assessments
Laboratory assessments (i.e., of serum, urine, and CSF), electrophysiological testing, and neuroimaging are frequently employed in the neuropsychiatric evaluation, particularly when the neuropsychiatric history and/or examination findings suggest the presence of a medical or neurological disorder for which such tests may be informative and/or potentially diagnostic. Although there remains a lack of consensus on the utility of screening laboratory measures in the evaluation of persons with neuropsychiatric disorders, the American Academy of Neurology accords the status of “guideline” to the asessement of B12 and thyroid-stimulating hormone (TSH) levels in the evaluation of persons with suspected dementias (26). However, clinical judgment based on the patient’s history and the examination findings remains the best guide to selection of laboratory measures.
Electrophysiological testing, including electroencephalography, is generally undertaken in outpatient evaluations only when epilepsy is suspected or when this testing is likely to provide data that will clarify the etiology of a confusional state.
There is considerable disagreement among professional associations and third-party payers regarding the indications for clinical neuroimaging in neuropsychiatry. However, Rauch and Renshaw (27) and Hurley et al. (28) described several clinical contexts in which neuroimaging may be appropriate. These include new-onset psychosis, dementia or clinically significant cognitive impairment, new-onset psychiatric symptoms in a patient age 50 years or older, neuropsychiatric symptoms associated with abnormal neurological signs or a history of brain injury, and catatonia. The American Academy of Neurology also accords the status of “guideline” to neuroimaging (computed tomography or magnetic resonance imaging) assessement of persons with suspected dementias (26). Magnetic resonance imaging (MRI) of the brain is used routinely for this purpose in the neuropsychiatric evaluation.
Evaluation Findings
The patient described in the clinical vignette underwent a comprehensive neuropsychiatric assessment, given that the accuracy of the diagnosis of late-onset bipolar disorder was in doubt. The clinical interview identified no significant prior developmental, medical, neurological, psychiatric, or substance use disorders; no family history of neuropsychiatric disturbance; and no significant psychosocial stressor before the onset of his neuropsychiatric condition at age 55 years. The patient’s history indicated that a persistent and progressive change in personality, behavior, and cognition began at that age; the former two changes were the earliest and remained the most prominent features of his condition. All of these disturbances worsened after the patient started treatment with lithium. His Neuropsychiatric Inventory score was 56 (abnormal), with the symptoms of severe apathy, disinhibition, affective lability (pathological laughing and crying), and aberrant motor behavior contributing most to this score. Impaired ability to maintain sleep at night was reported; however, the patient continued to need sleep, as evidenced by his frequent daytime napping. His appetite was not increased per se; instead, his food preferences were limited almost exclusively to sweets. It is important to note that the clinical interview clarified that the emotional disturbance experienced by this patient was one of affective expression alone, that it developed after several years of progressive neuropsychiatric decline, and that it did not occur in a temporally predictable manner with respect to his other neuropsychiatric symptoms. The results of the general physical examination were unremarkable, but the neurological examination revealed glabellar, snout, bilateral palmomental, and bilateral grasp reflexes, as well as paratonia. The findings on the general mental status examination were consistent with the patient’s Neuropsychiatric Inventory score. Cognitive assessment yielded an MMSE score of 24 of 30 and a Frontal Assessment Battery score of 10 of 18, which were 2.4 and 9.1 standard deviations below age-adjusted expectations, respectively. The results of the laboratory assessments, including serum B12 and TSH levels, were normal. As expected on the basis of the cognitive, emotional, behavioral, and neurological findings, an MRI of the brain showed marked bilateral anterior temporal and inferior frontal atrophy and mild-to-moderate bilateral dorsal and medial frontal atrophy (Figure 1).
The neuropsychiatric evaluation identified an acquired, persistent, and progressive deterioration of frontally and anterior temporally mediated cognitive, emotional, behavioral, and neurological functions that was consistent with a diagnosis of frontotemporal dementia (29). Treatment with lithium was discontinued, resulting in modest improvements in cognition and behavior. One month later, treatment with 25 mg/day of sertraline was initiated, and the dose was gradually increased to 100 mg/day. This intervention substantially reduced the severity of the patient’s pathological laughing and crying, impulsivity, and aberrant motor behavior (perseveration) and was without adverse effects on his cognition or neurological function.
Summary and Recommendations
Among older persons, symptoms such as impulsive, disinhibited, and socially inappropriate behavior may be attributable to late-onset bipolar disorder. However, attribution of such symptoms to bipolar disorder requires the occurrence of a pervasive and sustained disturbance in mood to which such symptoms are temporally related. The constellation of bipolar disorder symptoms among older adults is expected to be typical for this disorder (3–5), if perhaps on average of lesser severity than among younger persons (5), and to respond to standard pharmacological interventions (2). As illustrated in the case vignette, atypical clinical features and suboptimal treatment responses are strong indicators of the need to consider alternate diagnoses.
The higher frequency of neurological disorders among persons with late-onset bipolar disorder (2, 4) suggests the need for a thorough clinical evaluation and construction of a comprehensive differential diagnosis in all such cases. Use of a neuropsychiatric approach in the evaluation of persons with late-onset behavioral disturbances is recommended. This approach integrates key elements of the traditionally separate psychiatric and neurological interviews and examinations and facilitates comprehensive assessment of cognitive, emotional, behavioral, and sensorimotor functions. Cerebral neuroimaging, generally with MRI, is employed routinely in the neuropsychiatric evaluation of older adults. Data derived from the neuropsychiatric evaluation are interpreted first in terms of brain-behavior relationships (i.e., constructing an anatomy of illness) and then with respect to potential etiologies for the pattern of clinical findings. In the case presented here and in other cases in which clinical symptoms and/or treatment responses are atypical for primary psychiatric illnesses, a neuropsychiatric approach to clinical evaluation may clarify the diagnosis and improve patient care.
Received Nov. 28, 2005; accepted Nov. 28, 2005. From the Departments of Psychiatry and Neurology, University of Colorado School of Medicine; and the Brain Injury Rehabilitation Unit, HealthONE Spalding Rehabilitation Hospital, Aurora, Colo. Address correspondence and reprint requests to Dr. Arciniegas, Neuropsychiatry Service, Department of Psychiatry, University of Colorado School of Medicine, Campus Box C268-25, 4200 East Ninth Ave., Denver, CO 80262; [email protected] (e-mail).
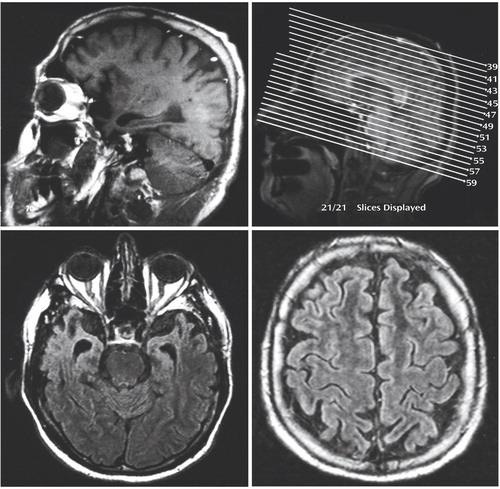
Figure 1. Magnetic Resonance Images of the Brain Showing Regional Atrophy in an Older Patient With Disturbance in Affective Expression Attributed to New-Onset Bipolar Disordera
aUpper left panel: T1-weighted sagittal image through the right hemisphere demonstrating marked inferior frontal and anterior temporal atrophy and mild dorsal frontal atrophy. Upper right panel: T1-weighted sagittal locator image. Lower left panel: T2-weighted fluid attenuated inversion recovery (FLAIR) image (at slice 53) demonstrating severe bilateral anterior temporal atrophy and anterior expansion of the temporal horns of the lateral ventricles in an ex vacuo manner, with relative preservation of medial temporal cortical volume. Lower right panel: T 2-weighted FLAIR image (at slice 43) of dorsal frontal areas anterior to the central sulcus demonstrating mild-to-moderate bilateral atrophy. No masses or other intracranial pathology is evident.
1. Sajatovic M, Blow FC, Ignacio RV, Kales HC: New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005; 13:282–289Crossref, Medline, Google Scholar
2. Depp CA, Jeste DV: Bipolar disorder in older adults: a critical review. Bipolar Disord 2004; 6:343–367Crossref, Medline, Google Scholar
3. Benazzi F: Bipolar II depression in late life: prevalence and clinical features in 525 depressed outpatients. J Affect Disord 2001; 66:13–18Crossref, Medline, Google Scholar
4. Almeida OP, Fenner S: Bipolar disorder: similarities and differences between patients with illness onset before and after 65 years of age. Int Psychogeriatr 2002; 14:311–322Crossref, Medline, Google Scholar
5. Depp CA, Jin H, Mohamed S, Kaskow J, Moore DJ, Jeste DV: Bipolar disorder in middle-aged and elderly adults: is age of onset important? J Nerv Ment Dis 2004; 192:796–799Crossref, Medline, Google Scholar
6. Meeks S: Bipolar disorder in the latter half of life: symptom presentation, global functioning and age of onset. J Affect Disord 1999; 52:161–167Crossref, Medline, Google Scholar
7. Chen ST, Altshuler LL, Melnyk KA, Erhart SM, Miller E, Mintz J: Efficacy of lithium versus valproate in the treatment of mania in the elderly: a retrospective study. J Clin Psychiatry 1999; 60:181–186Crossref, Medline, Google Scholar
8. Arciniegas DB, Topkoff J: The neuropsychiatry of pathologic affect: an approach to evaluation and treatment. Semin Clin Neuropsychiatry 2000; 5:290–306Crossref, Medline, Google Scholar
9. Arciniegas DB, Lauterbach EC, Anderson KE, Chow TW, Flashman LA, Hurley RA, Kaufer DI, McAllister TW, Reeve A, Schiffer RB, Silver JM: The differential diagnosis of pseudobulbar affect (PBA): distinguishing PBA among disorders of mood and affect. CNS Spectr 2005; 10:1–14Medline, Google Scholar
10. Wilson SAK: Some problems in neurology, II: pathological laughing and crying. J Neurol Psychopathol 1924; 4:299–333Crossref, Medline, Google Scholar
11. Poeck K: Pathological laughing and weeping in patients with progressive bulbar palsy. Ger Med Mon 1969; 14:394–397Medline, Google Scholar
12. Poeck K: Pathophysiology of emotional disorders associated with brain damage, in Handbook of Clinical Neurology, vol 3. Edited by Vinken PJ, Bruyn GW. Amsterdam, North Holland, 1969, pp 343-367Google Scholar
13. Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR: Pathological laughter and crying: a link to the cerebellum. Brain 2001; 124:1708–1719Crossref, Medline, Google Scholar
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
15. Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, Jablenski A, Regier D, Sartorius N: SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 1990; 47:589–593Crossref, Medline, Google Scholar
16. Swartz JR, Miller BL, Lesser IM, Booth R, Darby A, Wohl M, Benson DF: Behavioral phenomenology in Alzheimer’s disease, frontotemporal dementia, and late-life depression: a retrospective analysis. J Geriatr Psychiatry Neurol 1997; 10:67–74Crossref, Medline, Google Scholar
17. Damasceno A, Delicio AM, Mazo DF, Zullo JF, Scherer P, Ng RT, Damasceno BP: Primitive reflexes and cognitive function. Arq Neuropsiquiatr 2005; 63:577–582Crossref, Medline, Google Scholar
18. Benassi G, D’Alessandro R, Gallassi R, Morreale A, Lugaresi E: Neurological examination in subjects over 65 years: an epidemiological survey. Neuroepidemiology 1990; 9:27–38Crossref, Medline, Google Scholar
19. Di Legge S, Di Piero V, Altieri M, Vicenzini E, Tombari D, Di Stani F, Lenzi GL: Usefulness of primitive reflexes in demented and non-demented cerebrovascular patients in daily clinical practice. Eur Neurol 2001; 45:104–110Crossref, Medline, Google Scholar
20. Hogan DB, Ebly EM: Primitive reflexes and dementia: results from the Canadian Study of Health and Aging. Age Ageing 1995; 24:375–381Crossref, Medline, Google Scholar
21. Walterfang M, Velakoulis D: Cortical release signs in psychiatry. Aust NZ J Psychiatry 2005; 39:317–327Crossref, Medline, Google Scholar
22. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
23. Dubois B, Slachevsky A, Litvan I, Pillon B: The FAB: a frontal assessment battery at bedside. Neurol 2000; 55:1621–1626Crossref, Medline, Google Scholar
24. Kaye K, Grigsby J, Robbins LJ, Korzun B: Prediction of independent functioning and behavior problems in geriatric patients. J Am Geriatr Soc 1990; 38:1304–1310Crossref, Medline, Google Scholar
25. Spreen O, Strauss E: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 2nd ed. Oxford, UK, Oxford University Press, 1998Google Scholar
26. Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC: Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143–1153Crossref, Medline, Google Scholar
27. Rauch SL, Renshaw PF: Clinical neuroimaging in psychiatry. Harv Rev Psychiatry 1995; 2:297–312Crossref, Medline, Google Scholar
28. Hurley RA, Hayman LA, Taber KH: Clinical imaging in neuropsychiatry, in The American Psychiatric Publishing Textbook of Neuropsychiatry. Edited by Yudofsky SC, Hales RE. Washington, DC, American Psychiatric Publishing, 2002, pp 245-283Google Scholar
29. Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF: Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554Crossref, Medline, Google Scholar



