Brain Serotonin Transporter Distribution in Subjects With Impulsive Aggressivity: A Positron Emission Study With [11C]McN 5652
Abstract
OBJECTIVE: The serotonin system is believed to play a role in modulating impulsivity and violence. Previous imaging studies have implicated the anterior cingulate and orbitofrontal cortex in impulsive aggression. This study evaluated regional serotonin transporter distribution in the brain of individuals with impulsive aggression by using positron emission tomography (PET) with the serotonin transporter PET radiotracer [11C]McN 5652. METHOD: Ten individuals with impulsive aggression and 10 age- and sex-matched healthy comparison subjects underwent [11C]McN 5652 PET. All individuals were medication free at the time of scanning. Regional total distribution volumes were derived by using a one-tissue compartment kinetic model with arterial input function. Outcome measures of serotonin transporter availability included the binding potential and the specific-to-nonspecific partition coefficient (V3″). RESULTS: Serotonin transporter availability was significantly reduced in the anterior cingulate cortex of individuals with impulsive aggression compared with healthy subjects, as noted by differences in both binding potential (mean=3.1 ml/g [SD=1.9] versus 5.0 ml/g [SD=2.0], respectively) and V3″ (mean=0.15 [SD=0.09] versus 0.26 [SD=0.09]). In other regions examined, serotonin transporter density was nonsignificantly lower in individuals with impulsive aggression compared with healthy subjects. CONCLUSIONS: Pathological impulsive aggressivity might be associated with lower serotonergic innervation in the anterior cingulate cortex, a region that plays an important role in affective regulation.
Reduced activity of the serotonin (5-HT) system has been implicated in impulsive violence and aggression in studies that have used a variety of paradigms, including measurement of CSF serotonin metabolites, hormonal response to serotonergic probes, and imaging metabolic changes with serotonergic agents. In initial studies, reduced CSF concentration of the serotonin metabolite 5-hydroxyindoleacetic acid was demonstrated in individuals with a history of aggression (1, 2). Subsequent studies have linked this finding to impulsive aggression (3). Moreover, a blunted hormonal response to pharmacological manipulation of central serotonin function has been observed in personality disorder patients with impulsive aggression (4, 5).
Studies of brain lesions have pointed to the orbitofrontal cortex and the anterior cingulate gyrus as key areas regulating the generation of aggressive behaviors (6–10). Irritability and angry outbursts have been associated with orbitofrontal cortex damage in neurologic patients (11). Lesions of the medial orbital cortex early in childhood can result in antisocial, disinhibited, aggressive behavior later in life (12). These studies suggest that the orbital frontal and adjacent medial frontal cortex exert an inhibitory influence on aggressivity. Studies combining positron emission tomography (PET) imaging of regional glucose metabolism with pharmacologic challenges aimed at increasing serotonergic function also point toward altered 5-HT function in these regions. In healthy subjects, a single dose of the serotonin-releasing agent fenfluramine resulted in an increase in glucose metabolism in the orbitofrontal cortex and medial frontal and cingulate regions. Such an increase was not observed in subjects with impulsive aggression (13). Furthermore, patients with impulsive aggression demonstrated altered metabolic response to the serotonergic agent meta-chlorophenylpiperazine (m-CPP) in the orbitofrontal cortex and the anterior cingulate cortex (14). Together, these findings are consistent with the existence of reduced 5-HT function in the orbitofrontal cortex and anterior cingulate cortex in subjects with impulsive aggression.
In this study, we assess regional brain serotonin innervation in subjects with impulsive aggression by using in vivo imaging of serotonin transporter with PET and [11C](+)-6β-(4-methylthiophenyl)-1,2,3,5,6α,10β-hexahydropyrrolo[2,1-a]isoquinoline ([11C]McN 5652). This radiotracer has been successfully developed to image serotonin transporter density in humans (15–19) and has been used in a number of clinical studies, including studies of patients with mood disorders (20), obsessive-compulsive disorder (21), and ecstasy abuse (22, 23). Given that the strongest finding in our m-CPP study was reduced activation of the cingulate cortex (14), the main hypothesis of this study was that individuals with impulsive aggression would have reduced serotonin transporter density in the anterior cingulate cortex. Serotonin transporter density in the orbitofrontal cortex is too low to be accurately measured with PET and [11C]McN 5652 (18), so this region was not evaluated in this study.
Method
Human Subjects
The study was approved by the institutional review boards of the New York State Psychiatric Institute, Columbia Presbyterian Medical Center, Mount Sinai Hospital, and the Bronx Veterans Affairs Medical Center. Written informed consent was obtained from each subject after explanation of the study procedures. All subjects were free of significant medical problems, had no current or past neurological disorder, had no history of loss of consciousness, were not pregnant or nursing, and had not taken any psychoactive medications in the 3 weeks preceding the PET scan (6 weeks for fluoxetine). Ten patients (five men and five women; mean age=35 years [SD=9, range=18–51]) meeting criteria for intermittent explosive disorder–revised (impulsive aggression) (24) were recruited through advertisements in local newspapers and referrals from outpatient psychiatrists at the Bronx Veterans Affairs Medical Center and Mount Sinai School of Medicine. Patients were considered eligible for the impulsive aggression group if they met research diagnostic criteria for intermittent explosive disorder–revised (25) and the DSM-IV borderline personality disorder “impulsiveness” criterion or the borderline personality disorder “self-damaging” criterion according to a “Module for Intermittent Explosive Disorder-Revised” (prepared by Coccaro et al., personal communication). The Structured Interview for DSM-IV Personality Disorders (26) was used for personality disorder diagnoses. Patients with a history of schizophrenia or other psychotic disorder according to the Structured Clinical Interview for DSM-IV Axis I Disorders (27) were excluded. All subjects with a history of alcohol/drug dependence or substance abuse that had been active in the preceding 6 months were also excluded from the study. Ten healthy comparison subjects (five men and five women; mean age=34 years [SD=8, range=24–49]) with no current or past DSM-IV axis I psychiatric disorder were recruited through advertisements in local newspapers.
The absence of pregnancy and medical and neurological abnormalities was confirmed by a review of the patient’s history and systems, physical examination, routine blood tests including pregnancy test, urine toxicology, and electrocardiogram recordings.
Radiotracer
The standard (+)-McN 5652 was a gift from R.W. Johnson Pharmaceutical Research Institute. The precursor for the production of [11C] (+)-McN 5652, (+)-McN butyryl thioester tartrate, was prepared from (+)-McN 5652 by a modified literature procedure (28) as described previously (29). Radiochemical and chemical purity of [11C](+)-McN 5652 in saline was >95%.
PET Protocol
PET imaging was performed with the ECAT EXACT HR+ (Siemens/CTI, Knoxville, Tenn.). Sixty-three slices covered an axial field of view of 15.5 cm, axial sampling of 3.46 mm, three-dimensional mode in plane and axial resolution of 4.4 and 4.1 mm, respectively, full width at half maximum at the center of the field of view. An arterial catheter was inserted in the radial artery after completion of the Allen test and infiltration of the skin with 1% lidocaine. A venous catheter was inserted in a forearm vein on the opposite side. Head movement minimization was achieved with a polyurethane head immobilization system (Soule Medical, Tampa, Fla.) (30). A 10-minute transmission scan was obtained before radiotracer injection. [11C]McN 5652 was injected intravenously over 45 seconds. Emission data were collected in the three-dimensional mode for 120 minutes as 21 successive frames of increasing duration (three for 20 seconds, three for 1 minute, three for 2 minutes, two for 5 minutes, 10 for 10 minutes).
Input Function Measurement
Following radiotracer injection, arterial samples were collected every 10 seconds with an automated sampling system for the first 2 minutes, and manually thereafter at longer intervals. A total of 32 samples were obtained per scan. Seven samples (collected at 2, 16, 30, 50, 70, 90, and 120 minutes) were further processed by high-performance liquid chromatography to measure the fraction of plasma activity representing unmetabolized parent compound (18).
A biexponential function was fitted to the seven measured unmetabolized fractions, which was then used to interpolate values between the measurements. The smallest exponential of the unmetabolized fraction curve, λpar, was constrained to the difference between λcer, the terminal rate of washout of cerebellar activity, and λtot, the smallest elimination rate constant of the total plasma activity (31).
The input function was calculated as the product of total counts and interpolated unmetabolized fraction at each time point. The measured input function values (Ca(t) [mCi/ml]) were fitted to a sum of three exponentials from the time of peak plasma activity, and the fitted values were used as the input to the kinetic analysis. The initial distribution volume (Vbol [liters]) was calculated as the ratio of injected dose to peak plasma parent concentration. The clearance of the parent compound (CL [liters/hour]) was calculated as the ratio of the injected dose to the area under the curve of the input function (32).
The high retention (>90%) of free [11C]McN 5652 on the filter precludes the free fraction measurement of [11C]McN 5652; therefore, plasma f1 was not determined (18).
MRI Acquisition and Segmentation Procedures
MRIs, three-dimensional spoiled gradient-recall acquisition in the steady state, were acquired on a GE 1.5-T Signa Advantage system, as previously described (33). MRI segmentation was performed within MEDx (Sensor Systems, Inc., Sterling, Va.), with original subroutines implemented in MATLAB (The Math Works, Inc., Natick, Mass.). Steps for MRI segmentation included correction for field inhomogeneities, fitting of the intensity distribution to a sum of three Gaussian functions, voxel classification, and post filtering (34).
Image Analysis
Images were reconstructed to a 128×128 matrix (pixel size of 2.5×2.5 mm2). Reconstruction was performed with attenuation correction that used the transmission data and a Shepp 0.5 filter (cutoff 0.5 cycles/projection ray). Reconstructed image files were then processed with the image analysis software MEDx (Sensor Systems, Inc., Sterling, Va.). If indicated following visual inspection, frames were realigned to a frame of reference using a least-squares algorithm for within-modality coregistration (automated image registration) (35). The results of the frame-to-frame realignment were checked again visually. Following frame to frame registration, the 21 frames were summed to one dataset, which was coregistered to the MRI dataset using automated image registration (35). The spatial transformation derived from the summed PET registration procedure was then applied to each individual frame. Thus, each PET frame was resampled in the coronal plane to a voxel volume of 1.5×0.9×0.9 mm3.
Regions of interest (N=10) and region of reference (cerebellum) boundaries were drawn on the MRI according to criteria derived from brain atlases (36, 37) and published reports (38–41). Analysis was restricted to regions of interest where serotonin transporter density is high enough to provide a reliable signal. Regions of interest included the midbrain (encompassing serotonin transporter dense structures such as the raphe nuclei, substantia nigra, locus ceruleus, ventral tegmental area, and superior and inferior colliculi), thalamus, dorsal caudate, dorsal putamen, ventral striatum, amygdala, entorhinal cortex, hippocampus, parahippocampal gyrus, and the anterior cingulate cortex. A segmentation-based method was used for the neocortical regions, and a direct identification method was used for the subcortical regions (33). For bilateral regions, right and left values were averaged. The contribution of plasma total activity to the regional activity was calculated assuming a 5% blood volume in the regions of interest (42), and tissue activities were calculated as the total regional activities minus the plasma contribution.
Derivation of Distribution Volumes
Derivation of [11C]McN 5652 regional tissue distribution volumes was performed with kinetic modeling using the arterial input function and a one-tissue compartment model. This model has been demonstrated to provide reliable estimates of total distribution volume for [11C]McN 5652 (16, 18, 19). Total distribution volume (VT [ml/g]), which is equal to the ratio of tissue to plasma parent activity at equilibrium, was derived as the K1/k2 ratio, where K1 (ml/g/min) and k2 (1/min) are the unidirectional fractional rate constants for the transfer of the tracer in and out of the brain, respectively (43, 44). Kinetic parameters were derived by nonlinear regression using a Levenberg-Marquart least-squares minimization procedure (45) implemented in MATLAB (The Math Works, Inc., South Natick, Mass.) as previously described (43). Given the unequal sampling over time (increasing frame acquisition time from the beginning to the end of the study), the least-squares minimization procedure was weighted by the frame acquisition time.
Serotonin Transporter Parameters
Derivation of serotonin transporter parameters was based upon the following assumptions: 1) given the negligible density of serotonin transporter in the cerebellum (46–48), cerebellum total distribution volume was assumed to be representative of equilibrium nonspecific binding; 2) the nonspecific binding did not vary significantly between regions.
Two measures of serotonin transporter availability were calculated. The binding potential (ml/g) was derived as the difference in total distribution volume between the region of interest and the cerebellum, the reference region. The relationship between binding potential and serotonin transporter receptor parameters is given by (49)
where Bmax is the regional concentration of serotonin transporter (nmol/liter), and KD is the in vivo affinity of the tracer for serotonin transporter (nmol/liter).
The specific-to-nonspecific equilibrium partition coefficient (V3″ [unitless]) was derived as the ratio of binding potential to total distribution volume in the cerebellum. The relationship between V3″ and serotonin transporter receptor parameters is given by (49)
where f2 is the free fraction of the nonspecific distribution volume in the brain (f2=f1/ cerebellum VT).
Statistical Analysis
Between-group comparisons were assessed with unpaired two-tailed t tests with a probability of 0.05 set as the level of significance. The a priori hypothesis of this study related to serotonin transporter level in the anterior cingulate cortex, and therefore no correction for multiple comparisons was employed in the examination of this brain region. The other brain regions were also analyzed for between-group differences to determine the specificity of the difference seen in the anterior cingulate cortex. In order to correct for multiple comparisons, these analyses employed a univariate repeated-measures analysis of variance (ANOVA) with brain regions as the within-subject factor and diagnosis as the between-subject factor.
Results
Group Composition
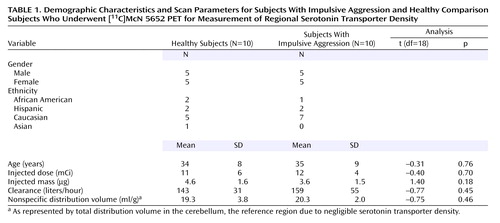
Demographic data for study participants are shown in Table 1. No significant group differences were observed on any demographic factor. One individual in the impulsive aggression group reported taking fluoxetine for 5 months several years before the study; all other participants were medication naive at the time of the scan. A significant degree of psychiatric comorbidity was present in the impulsive aggression group. This was particularly evident with regard to axis II diagnoses. All subjects had at least one personality disorder, the most common being borderline personality disorder (N=7), followed by paranoid (N=4), narcissistic (N=3), obsessive-compulsive (N=4), schizotypal (N=2), antisocial (N=2), histrionic (N=2), dependent (N=1), and avoidant (N=1) personality disorders. At the time of scanning, no subject had an active axis I mood disorder. However, several subjects were retrospectively diagnosed with comorbid mood illness. These included major depressive disorder (N=5), dysthymia (N=1), and bipolar II disorder (N=1). Anxiety disorders were also prevalent in this group, such as generalized anxiety disorder (N=1), adjustment disorder (N=1), social phobia (N=1), obsessive-compulsive disorder (N=1), and body dysmorphic disorder (N=1). In addition, there were two subjects with a history of alcohol dependence and two with a history of alcohol abuse; all had been in remission for greater than 6 months.
Scan Parameters
Scan parameters, including the injected dose, injected mass, and specific activity, did not differ between groups (Table 1). The plasma clearances of [11C]McN 5652 were similar between the groups. No significant group difference was observed in [11C]McN 5652 nonspecific distribution volume, measured as the cerebellar distribution volume.
Regional Volumes
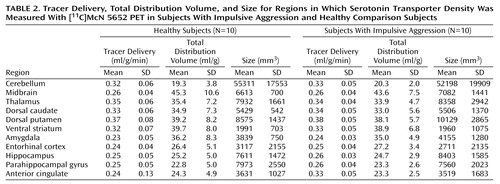
Table 2 lists the average size for each region of interest in mm3. There was no difference in anterior cingulate cortex volume between the groups (t=0.18, df=18, p=0.86). For the other regions, a repeated-measures ANOVA showed a significant effect of region (F=121.4, df=1, 21, p<0.0001), no effect of group (F=0.04, df=1, 18, p=0.85), and no group-by-region interaction (F=0.23, df=1, 21, p=0.99).
Region of Interest Kinetic Analysis
After injection, the accumulation of [11C]McN 5652 activity was consistent with the known distribution of serotonin transporter in the brain (Figure 1). The kinetic analysis converged in all regions for all subjects. Regional values of the unidirectional fractional rate constant of tracer delivery as well as the regional total distribution volume values are shown in Figure 2. There was no difference between groups in the tracer delivery values for the anterior cingulate cortex (t=–0.52, df=18, p=0.14). Examining all regions, a significant region effect was observed (repeated-measures ANOVA: F=153.0, df=1, 21, p<0.0001), with no difference between groups (repeated-measures ANOVA: F=0.06, df=1, 18, p=0.81) and no group-by-region interaction (repeated-measures ANOVA: F=0.635, df=1, 21, p=0.80). Similarly, for total distribution volume, no group differences were present in the anterior cingulate cortex (t=0.53, df=18, p=0.60). For the other regions, a region effect was observed (repeated-measures ANOVA: F=152.1, df=1, 21, p<0.0001), with no effect of group (repeated-measures ANOVA: F=0.09, df=1, 18, p=0.77) and no group-by-region interaction (repeated-measures ANOVA: F=0.60, df=1, 21, p=0.83).
[11C]McN 5652 Binding Potential
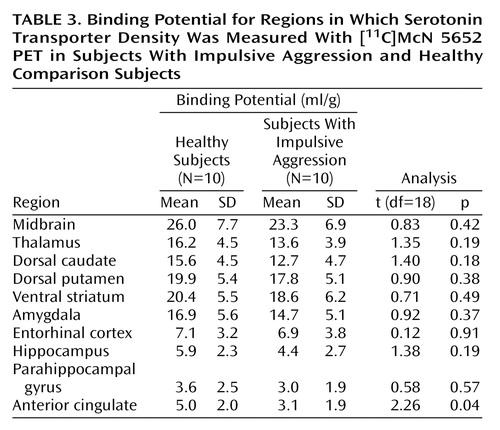
As shown in Table 3, a significant difference in binding potential between healthy subjects and subjects with impulsive aggression was revealed in the anterior cingulate cortex. For all other regions, no group differences were detected (repeated-measures ANOVA: F=1.24, df=1, 18, p=0.28). A significant regional effect was seen (F=137.1, df=1, 21, p<0.0001), with no group-by-region interaction (F=0.48, df=1, 21, p=0.90).
[11C]McN 5652 Regional V3″
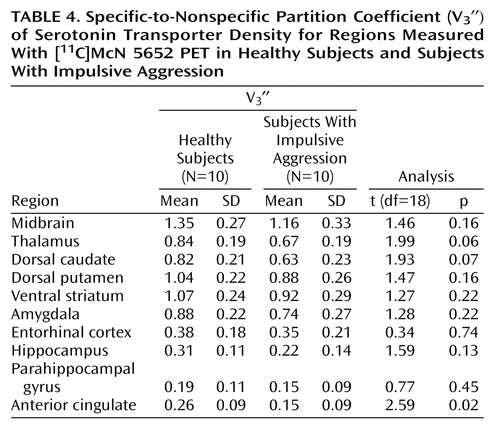
As shown in Table 4, a significant difference in V3″ between healthy subjects and subjects with impulsive aggression was revealed in the anterior cingulate cortex. Examination of all regions in a repeated-measure design resulted in no significant difference between the groups (F=2.83, df=1, 18, p=0.11), with a significant regional difference (F=163.6, df=1, 21, p<0.0001) and no region-by-group interaction (F=1.0, df=1, 21, p=0.43). The effect size of the reduction in [11C]McN 5652 V3″ in all regions examined is presented in Figure 2.
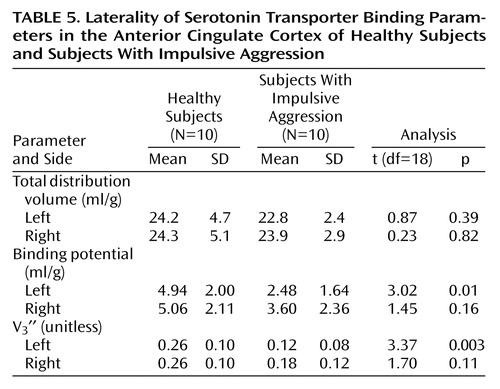
Laterality
Given the finding of reduced serotonin transporter parameters in the anterior cingulate cortex of patients relative to healthy comparison subjects, we performed a post hoc analysis to determine if this finding was specific to the left or right hemisphere. Table 5 shows the results of this analysis. No difference was seen in total distribution volume in either the right or left anterior cingulate cortex in patients relative to healthy subjects. However, for the outcome measures binding potential and V3″, patients with impulsive aggression had significantly lower values in the left anterior cingulate cortex than did the healthy subjects, whereas no significant difference was seen in the right anterior cingulate cortex. This finding was not due to differences between the right and left anterior cingulate cortex in healthy subjects, since total distribution volume, binding potential, and V3″ values were not different in the left relative to the right anterior cingulate cortex. For patients with impulsive aggression, no differences in total distribution volume were seen when comparing the left anterior cingulate cortex with the right anterior cingulate cortex. However, for binding potential and V3″, the values in the left anterior cingulate cortex tended to be lower than the right anterior cingulate cortex (paired t test, p=0.06). No significant differences were observed for any other region in this analysis.
Discussion
This study suggests that pathological impulsive aggressivity is associated with a reduction in serotonin transporter availability in the anterior cingulate cortex, a reduction that might reflect reduced 5-HT innervation. Although not statistically significant, our data also suggest a modest decrease in serotonin transporter availability in other regions.
The use of a fully quantitative imaging method was a strength of this study. The kinetic analysis is not affected by potential group differences in radiotracer plasma clearance or regional cerebral blood flow (50). Measurement of the arterial input function enabled the quantitative derivation of distribution volumes, from which both binding potential and V3″ were calculated. Although methods for deriving [11C]McN 5652 V3″ without arterial sampling have been proposed (20), only with the arterial input function could we demonstrate the absence of group differences in cerebellum distribution volumes and thus validate the use of V3″ for between-group comparisons of receptor parameters. In the absence of group differences in cerebellum distribution volumes, results derived with binding potential and V3″ (i.e., binding potential normalized by the nonspecific distribution volume) are essentially similar, which was the case here.
This study has several limitations. The small size of this study group was adequate to detect the relatively large decrease in serotonin transporter in the anterior cingulate cortex in subjects with impulsive aggression but limited our power to detect potential significant differences in other regions. As described, in all regions, V3″ values were lower for the subjects with impulsive aggression than the healthy subjects. Analysis of a larger group would be required to further explore this difference. Examination of the overall effect size for the repeated-measures ANOVA reveals that approximately 30 individuals per group would be required to detect this difference at the level of p<0.05. The effect size (d) for the differences in the individual regions ranged from 1.22 in the anterior cingulate cortex to 0.16 in the entorhinal cortex.
Another limitation of this study arises from the use of [11C]McN 5652 to measure serotonin transporter. This radiotracer is associated with high levels of nonspecific binding, limiting the ability to quantify serotonin transporter in regions of low serotonin transporter density such as the neocortex (18). The ability to detect differences in these regions will likely be enhanced by the use of newly developed radiotracers for serotonin transporter such as [11C]3-amino-4-[2-[(dimethylamino)methyl]phenylthio) benzonitrile ([11C]DASB) (51) and [11C]2-[2-(dimethylaminomethylphenylthio)]-5-fluoromethylphenylamine ([11C]AFM) (52, 53). Both tracers markedly improve the signal-to-noise ratio compared with [11C]McN 5652 (29, 53).
Given previous studies implicating the anterior cingulate cortex in impulsive aggression, this area was the primary focus of our study. The finding of a reduction in serotonin transporter density in this region is consistent with results from studies of cerebral metabolism in impulsive aggression. In response to serotonergic challenges, including d,l-fenfluramine and m-CPP, the relative glucose metabolic rate of individuals with impulsive aggression is blunted in the anterior cingulate cortex relative to healthy subjects (13, 14). Fenfluramine acts by causing a direct release of serotonin and antagonizing its reuptake, whereas m-CPP acts as a partial agonist at the postsynaptic 5-HT2A and 5-HT2C receptors. On the basis of these mechanisms of action alone it is not possible to comment on the locus of the abnormality in impulsive aggression, i.e., whether the blunted response is secondary to a pre- or postsynaptic problem. Our study provides some insight into this question, suggesting that a presynaptic deficiency in serotonin innervation exists in the anterior cingulate cortex in this disorder. This result is in line with the observations from studies of serotonin metabolites that have linked reduced serotonin markers with impulsive aggression (1, 3, 54). Our findings are also in agreement with the postmortem finding of a decrease in serotonin transporter binding in suicide victims (55), viewed as a specific form of self-directed impulsive/aggressive behavior, as well as the finding of reduced platelet serotonin transporter in aggression (56).
Davidson et al. (57) proposed that disruption of the normal regulation of emotion–which involves several interconnected brain regions including the orbitofrontal cortex, amygdala, and anterior cingulate cortex—plays a role in the generation of violence. The anterior cingulate cortex can be separated into a dorsal “cognitive” portion and a rostral-ventral “affective” region (58). Evidence from a variety of domains indicates that the affective subdivision of the anterior cingulate cortex regulates the intensity of response to emotional stimuli. For example, stimulation of this area in animal models increases the latency of attack behavior (59). In humans, PET studies of cerebral blood flow demonstrate activation of the ventral anterior cingulate cortex when anger is induced in healthy men using imagery (60). This same area is activated when symptoms are provoked in individuals with simple phobia, OCD, or PTSD (61–63). Further evidence that the rostral-ventral anterior cingulate cortex plays a role in emotional regulation comes from the finding that this area was activated when men attempted to suppress sexual arousal in response to erotic film excerpts but not in the nonsuppression condition (64). Given the aforementioned limitations of the radiotracer, we were unable to separate the anterior cingulate cortex into the dorsal and rostral-ventral components. However, we did examine the laterality of our finding. Previously, there have been no reports of aggression-related laterality in the anterior cingulate cortex, although work by our group has demonstrated blunted metabolic response to m-CPP in the left orbitofrontal cortex (14). The results of this study, indicating a left-sided predominance of the abnormality, are consistent with findings showing that traumatic brain lesions to the left frontal cortex give rise to aggression and hostility, whereas right-sided lesions lead to anxiety/depression (8).
Our finding of an abnormality of the serotonergic innervation in the anterior cingulate cortex in subjects with impulsive aggression is consistent with the hypothesis that alterations in the normal function of this area may lead to difficulties with affect and impulse modulation, resulting in increased impulsivity and aggression.
Conclusions
This study detected a significant difference in anterior cingulate cortex serotonin transporter availability in individuals with impulsive aggression. These individuals react aggressively in response to emotionally laden interpersonal stimuli, such as conflict or perceived disrespect. This is hypothesized to result from impaired regulation of negative emotions, believed to be one of the primary functions of the anterior cingulate cortex (57). Future work extending the results of this study to other brain regions involved in the regulation of emotion and behavioral responses, such as the orbitofrontal cortex, is warranted to further characterize alterations of 5-HT function in impulsive aggressivity.
 |
 |
 |
 |
 |
Received Feb. 12, 2004; revision received April 23, 2004; accepted May 14, 2004. From the Departments of Psychiatry and Radiology, Columbia University College of Physicians and Surgeons and the New York State Psychiatric Institute; the Psychiatric Service, Bronx Veterans Affairs Medical Center, Bronx, N.Y.; and the Department of Psychiatry, Mount Sinai School of Medicine, New York. Address correspondence and reprint requests to Dr. Frankle, New York State Psychiatric Institute, 1051 Riverside Dr., Box 31, New York, NY 10032; [email protected] (e-mail). Ms. Curry died in June 2004. The authors dedicate this paper in memory of her.Supported in part by NIMH grants 1RO1 MH-63875, 5RO1 MH-56606, and 1-K02 MH-01603-01). The authors thank Julie Arcement, Jennifer Bae, Ashlie Darr, Ingrid Gelbard-Strokes, Elizabeth Hackett, Kimchung Ngo, Chaka Peters, Nurat Quadri, Celeste Reinking, Norman Simpson, Lyudmila Savenkova, Kris Wolff, and Zohar Zephrani for their technical assistance.
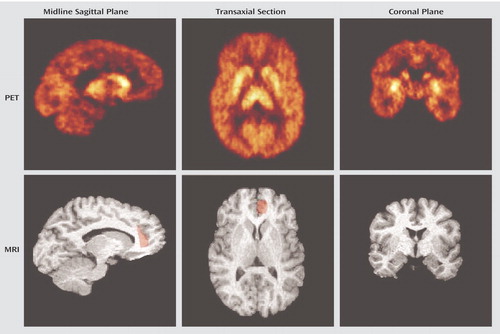
Figure 1. PET and MRI Images of Serotonin Transporter Distribution in a 37-Year-Old Woman With Impulsive Aggressiona
aThe PET images represent the activity from 40 to 90 minutes after injection of 13.76 mCi of [11C]McN 5652. Accumulation of activity can be seen in the thalamus and caudate in the midline sagittal plane images on the left. The region of interest for the anterior cingulate cortex is shaded in red on both the sagittal and transaxial MRI slices. The transaxial section demonstrates the high level of serotonin transporter in the striatum. The coronal plane images on the right show the ventral-dorsal serotonin transporter gradient in the striatum; the anterior cingulate cortex is not visible in the coronal section at this level.
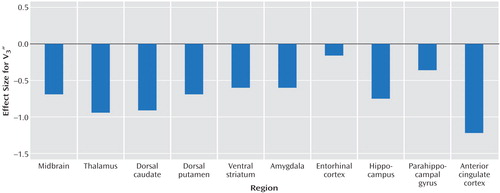
Figure 2. Effect Size of the Differences in Specific-to-Nonspecific Partition Coefficient (V3″) of Serotonin Transporter Density Between Healthy Subjects and Subjects With Impulsive Aggressiona
aValues are Cohen’s effect size (d) for unpaired t test, N=10 per group.
1. Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF: Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1979; 1:131–139Crossref, Medline, Google Scholar
2. Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK: Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am J Psychiatry 1982; 139:741–746Link, Google Scholar
3. Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK: Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 1983; 33:2609–2614Crossref, Medline, Google Scholar
4. Coccaro EF, Siever LJ, Klar HM, Maurer G, Cochrane K, Cooper TB, Mohs RC, Davis KL: Serotonergic studies in patients with affective and personality disorders: correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry 1989; 46:587–599Crossref, Medline, Google Scholar
5. Siever L, Trestman RL: The serotonin system and aggressive personality disorder. Int Clin Psychopharmacol 1993; 8(suppl 2):33–39Google Scholar
6. Weiger WA, Bear DM: An approach to the neurology of aggression. J Psychiatr Res 1988; 22:85–98Crossref, Medline, Google Scholar
7. Heinrichs RW: Frontal cerebral lesions and violent incidents in chronic neuropsychiatric patients. Biol Psychiatry 1989; 25:174–178Crossref, Medline, Google Scholar
8. Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM: Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology 1996; 46:1231–1238Crossref, Medline, Google Scholar
9. Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR: Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci 1999; 2:1032–1037Crossref, Medline, Google Scholar
10. Butter CM, Snyder DR, McDonald JA: Effects of orbital frontal lesions on aversive and aggressive behaviors in rhesus monkeys. J Comp Physiol Psychol 1970; 72:132–144Crossref, Medline, Google Scholar
11. Weiger WA, Bear DM: An approach to the neurology of aggression. J Psychiatr Res 1988; 22:85–98Crossref, Medline, Google Scholar
12. Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR: Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci 1999; 2:1032–1037Crossref, Medline, Google Scholar
13. Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA, Sevin E, Nunn M, Mitropoulou V: d,l-Fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology 1999; 20:413–423Crossref, Medline, Google Scholar
14. New AS, Hazlett EA, Buchsbaum MS, Goodman M, Reynolds D, Mitropoulou V, Sprung L, Shaw RB Jr, Koenigsberg H, Platholi J, Silverman J, Siever LJ: Blunted prefrontal cortical 18fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Arch Gen Psychiatry 2002; 59:621–629Crossref, Medline, Google Scholar
15. Suehiro M, Scheffel U, Ravert HT, Dannals RF, Wagner H Jr: [11C](+)McN5652 as a radiotracer for imaging serotonin uptake sites with PET. Life Sci 1993; 53:883–892Crossref, Medline, Google Scholar
16. Szabo Z, Scheffel U, Mathews WB, Ravert HT, Szabo K, Kraut M, Palmon S, Ricaurte GA, Dannals RF: Kinetic analysis of [11C]McN5652: a serotonin transporter radioligand. J Cereb Blood Flow Metab 1999; 19:967–981Crossref, Medline, Google Scholar
17. Szabo Z, Kao PF, Scheffel U, Suehiro M, Mathews WB, Ravert HT, Musachio JL, Marenco S, Kim SE, Ricaurte GA, et al: Positron emission tomography imaging of serotonin transporters in the human brain using [11C](+)McN5652. Synapse 1995; 20:37–43Crossref, Medline, Google Scholar
18. Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A, Mawlawi O, Slifstein M, Van Heertum RL, Mann JJ, Laruelle M: In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med 2000; 41:1465–1477Medline, Google Scholar
19. Buck A, Gucker PM, Schonbachler RD, Arigoni M, Kneifel S, Vollenweider FX, Ametamey SM, Burger C: Evaluation of serotonergic transporters using PET and [11C](+)McN-5652: assessment of methods. J Cereb Blood Flow Metab 2000; 20:253–262Crossref, Medline, Google Scholar
20. Ichimiya T, Suhara T, Sudo Y, Okubo Y, Nakayama K, Nankai M, Inoue M, Yasuno F, Takano A, Maeda J, Shibuya H: Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+)McN5652. Biol Psychiatry 2002; 51:715–722Crossref, Medline, Google Scholar
21. Simpson HB, Lombardo I, Slifstein M, Huang HY, Hwang DR, Abi-Dargham A, Liebowitz MR, Laruelle M: Serotonin transporters in obsessive-compulsive disorder: a positron emission tomography study with [(11)C]McN 5652. Biol Psychiatry 2003; 54:1414–1421Crossref, Medline, Google Scholar
22. McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA: Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet 1998; 352:1433–1437Crossref, Medline, Google Scholar
23. Buchert R, Thomasius R, Nebeling B, Petersen K, Obrocki J, Jenicke L, Wilke F, Wartberg L, Zapletalova P, Clausen M: Long-term effects of “Ecstasy” use on serotonin transporters of the brain investigated by PET. J Nucl Med 2003; 44:375–384Medline, Google Scholar
24. Coccaro EF, Kavoussi RJ, Berman ME, Lish JD: Intermittent explosive disorder-revised: development, reliability, and validity of research criteria. Compr Psychiatry 1998; 39:368–376Crossref, Medline, Google Scholar
25. Coccaro EF: Intermittent explosive disorder. Curr Psychiatry Rep 2000; 2:67–71Crossref, Medline, Google Scholar
26. Pfohl B, Blum N, Zimmerman M: Structured Interview for DSM-IV Personality: SIDP-IV. Washington, DC, American Psychiatric Press, 1997Google Scholar
27. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
28. Zessin J, Gucker P, Ametamey S, Steinback J, Brust P, Vollenweider F, Johannsen B, Schubiger P: Efficient synthesis of the enantiomerically pure thioester precursors of [11C]McN-5652 from racemic McN-5652. J Labelled Comp Radiopharm 1999; 42:1301–1312Crossref, Google Scholar
29. Frankle W, Huang Y, Hwang D-R, Talbot PS, Slifstein M, Van Heertum R, Abi-Dargham A, Laruelle M: Comparative evaluation of serotonin transporter radioligands [11C]DASB and [11C]McN 5652 in healthy humans. J Nucl Med 2004; 45:682–694Medline, Google Scholar
30. Mawlawi OM, Weiss R, Shinn A, Pidcock J, Slifstein M, Laruelle M: Performance characteristics of a head immobilization device for PET imaging (abstract). J Nucl Med 1999; 40:281PGoogle Scholar
31. Abi-Dargham A, Simpson N, Kegeles L, Parsey R, Hwang DR, Anjilvel S, Zea-Ponce Y, Lombardo I, Van Heertum R, Mann JJ, Foged C, Halldin C, Laruelle M: PET studies of binding competition between endogenous dopamine and the D1 radiotracer [11C]NNC 756. Synapse 1999; 32:93–109Crossref, Medline, Google Scholar
32. Abi-Dargham A, Laruelle M, Seibyl J, Rattner Z, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Bremner JD, Hyde TM, Charney DS, Hoffer PB, Innis RB: SPECT measurement of benzodiazepine receptors in human brain with [123-I]iomazenil: kinetic and equilibrium paradigms. J Nucl Med 1994; 35:228–238Medline, Google Scholar
33. Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M: Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 2002; 22:3708–3719Crossref, Medline, Google Scholar
34. Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, Anjilvel S, Pidcock J, Guo NN, Lombardo I, Mann JJ, Van Heertum R, Foged C, Halldin C, Laruelle M: Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab 2000; 20:225–243Crossref, Medline, Google Scholar
35. Woods RP, Cherry SR, Mazziotta JC: Rapid automated algorithm for aligning and reslicing PET images. J Comp Assist Tomogr 1992; 16:620–633Crossref, Medline, Google Scholar
36. Duvernoy H: The Human Brain: Surface, Three-Dimensional Sectional Anatomy and MRI. New York, Springer-Verlag Wien, 1991Google Scholar
37. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
38. Killiany RJ, Moss MB, Nicholson T, Jolez F, Sandor T: An interactive procedure for extracting features of the brain from magnetic resonance images: the lobes. Hum Brain Mapp 1997; 5:355–363Crossref, Medline, Google Scholar
39. Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL: Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatr Res Neuroimaging 1997; 75:31–48Crossref, Medline, Google Scholar
40. Pani L, Gessa GL, Carboni S, Portas CM, Rossetti ZL: Brain dialysis and dopamine: does the extracellular concentration of dopamine reflect synaptic release? Eur J Pharmacol 1990; 180:85–90Crossref, Medline, Google Scholar
41. Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M: Imaging human mesolimbic dopamine transmission with positron emission tomography, I: accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21:1034–1057Crossref, Medline, Google Scholar
42. Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ: A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol 1984; 15:217–227Crossref, Medline, Google Scholar
43. Laruelle M, Baldwin RM, Rattner Z, Al-Tikriti MS, Zea-Ponce Y, Zoghbi SS, Charney DS, Price JC, Frost JJ, Hoffer PB, Innis RB: SPECT quantification of [123I]iomazenil binding to benzodiazepine receptors in nonhuman primates, I: kinetic modeling of single bolus experiments. J Cereb Blood Flow Metab 1994; 14:439–452Crossref, Medline, Google Scholar
44. Koeppe RA, Holthoff VA, Frey KA, Kilbourn MR, Kuhl DE: Compartmental analysis of [11C]flumazenil kinetics for the estimation of ligand transport rate and receptor distribution using positron emission tomography. J Cereb Blood Flow Metab 1991; 11:735–744Crossref, Medline, Google Scholar
45. Levenberg K: A method for the solution of certain problems in least squares. Q Applied Mathematics 1944; 2:164–168Crossref, Google Scholar
46. Laruelle M, Vanisberg M, Maloteaux J: Regional and subcellular localization in human brain of [3H]paroxetine binding, a marker of serotonin uptake sites. Biol Psychiatry 1988; 24:299–309Crossref, Medline, Google Scholar
47. Backstrom I, Bergstrom M, Marcusson J: High affinity [3H]paroxetine binding to serotonin uptake sites in human brain tissue. Brain Res 1989; 486:261–268Crossref, Medline, Google Scholar
48. Plenge P, Mellerup ET, Laursen H: Regional distribution of the serotonin transport complex in human brain, identified with 3H-paroxetine, 3H-citalopram and 3H-imipramine. Prog Neuropsychopharmacol Biol Psychiatry 1990; 14:61–72Crossref, Medline, Google Scholar
49. Laruelle M, van Dyck C, Abi-Dargham A, Zea-Ponce Y, Zoghbi SS, Charney DS, Baldwin RM, Hoffer PB, Kung HF, Innis RB: Compartmental modeling of iodine-123-iodobenzofuran binding to dopamine D2 receptors in healthy subjects. J Nucl Med 1994; 35:743–754Medline, Google Scholar
50. Slifstein M, Laruelle M: Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol 2001; 28:595–608Crossref, Medline, Google Scholar
51. Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S: Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of (11)C-labeled 2-(phenylthio)araalkylamines. J Med Chem 2000; 43:3103–3110Crossref, Medline, Google Scholar
52. Huang Y, Bae S-A, Zhu Z, Guo N, Hwang DR, Laruelle M: Fluorinated analogues of ADAM as new PET radioligands for the serotonin transporter: synthesis and pharmacological evaluation. J Labelled Comp Radiopharm 2001; 44:S18-S20Google Scholar
53. Huang Y, Hwang DR, Narendran R, Sudo Y, Chatterjee R, Bae SA, Mawlawi O, Kegeles LS, Wilson AA, Kung HF, Laruelle M: Comparative evaluation in nonhuman primates of five PET radiotracers for imaging the serotonin transporters: [11C]McN 5652, [11C]ADAM, [11C]DASB, [11C]DAPA, and [11C]AFM. J Cereb Blood Flow Metab 2002; 22:1377–1398Crossref, Medline, Google Scholar
54. Asberg M, Traskman L, Thoren P: 5-HIAA in the cerebrospinal fluid: a biochemical suicide predictor? Arch Gen Psychiatry 1976; 33:1193–1197Crossref, Medline, Google Scholar
55. Arango V, Underwood MD, Gubbi AV, Mann JJ: Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res 1995; 688:121–133Crossref, Medline, Google Scholar
56. Brown CS, Kent TA, Bryant SG, Gevedon RM, Campbell JL, Felthous AR, Barratt ES, Rose RM: Blood platelet uptake of serotonin in episodic aggression. Psychiatry Res 1989; 27:5–12Crossref, Medline, Google Scholar
57. Davidson RJ, Putnam KM, Larson CL: Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 2000; 289:591–594Crossref, Medline, Google Scholar
58. Bush G, Luu P, Posner MI: Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4:215–222Crossref, Medline, Google Scholar
59. Siegel A, Edinger HM: Role of the limbic system in hypothalamically elicited attack behavior. Neurosci Biobehav Rev 1983; 7:395–407Crossref, Medline, Google Scholar
60. Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Lasko M, Macklin ML, Fischman AJ, Rauch SL: Anger in healthy men: a PET study using script-driven imagery. Biol Psychiatry 1999; 46:466–472Crossref, Medline, Google Scholar
61. Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ: Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry 1994; 51:62–70Crossref, Medline, Google Scholar
62. Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK: A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry 1996; 53:380–387Crossref, Medline, Google Scholar
63. Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L, Breiter HC, Fischman AJ, Manzo PA, Moretti C, Jenike MA: A positron emission tomographic study of simple phobic symptom provocation. Arch Gen Psychiatry 1995; 52:20–28Crossref, Medline, Google Scholar
64. Beauregard M, Levesque J, Bourgouin P: Neural correlates of conscious self-regulation of emotion. J Neurosci 2001; 21:RC165Google Scholar



