Aberrant Intracellular Calcium Signaling in Olfactory Neurons From Patients With Bipolar Disorder
Abstract
OBJECTIVE: The authors examined the feasibility of using olfactory receptor neurons from living patients to test whether calcium signaling is altered in a neuronal cell population in bipolar disorder. METHOD: Ratiometric fluorescence photomicroscopy was used to assess basal and stimulus-induced changes in intracellular calcium levels in biopsy-derived olfactory receptor neurons from seven euthymic patients with bipolar disorder who were medication-free, 10 euthymic patients with bipolar disorder who were treated with mood stabilizers, and 17 age- and sex-matched comparison subjects without bipolar disorder. RESULTS: Olfactory receptor neurons from the seven medication-free patients responded to stimuli predominantly with decreases in intracellular calcium, unlike those from the seven matched healthy subjects. Olfactory receptor neurons from patients treated with mood stabilizers were less likely to respond to stimulation than olfactory receptor neurons from medication-free patients. CONCLUSIONS: This study demonstrates the feasibility of using olfactory receptor neurons to examine alterations in intracellular signaling in neuronal cells from living patients. Our results, although based on a small number of subjects, suggest that altered intracellular calcium signaling in olfactory receptor neurons may be a trait of bipolar disorder.
Studies employing calcium-sensitive dyes have demonstrated that levels of both basal and agonist-stimulated intracellular calcium are significantly higher in blood cells from untreated manic and depressed patients with bipolar disorder than in those from healthy comparison subjects (1). In addition, this enhanced calcium response was reduced to the level of healthy comparison subjects following mood stabilizer treatment (2). An important question is whether these findings in peripheral blood cells can be extended to neurons from living subjects with the illness.
To answer this question, we explored the feasibility of using human olfactory receptor neurons to study alterations in signal transduction, particularly intracellular calcium, in patients with bipolar disorder. Intracellular calcium in olfactory receptor neurons was monitored under basal conditions and under stimulation by odorant mixture A and odorant mixture B. Mixture A odorants specifically elicit increases in cAMP in isolated cilia from rat olfactory receptor neurons, and mixture B odorants elicit little change in cAMP but elevate inositol 1,4,5-trisphosphate levels. Although it remains unclear whether there are two distinct odorant signaling pathways or one in rodent olfactory receptor neurons, we have demonstrated that these stimuli trigger calcium responses in human olfactory receptor neurons through at least two pharmacologically distinct mechanisms (3).
Method
Seven patients with bipolar disorder who had been free of psychotropic medication for at least 6 weeks, 10 patients with bipolar disorder who had been treated with therapeutic blood levels of either lithium or valproic acid for at least 3 weeks, and 17 age- and sex-matched healthy comparison subjects were recruited for olfactory epithelial biopsy (4)). Screening evaluation was conducted with the patient and nonpatient editions of the Structured Clinical Interview for DSM-IV. All subjects provided written informed consent after the procedure was fully explained. All patients were euthymic, defined in this study as a Young Mania Rating Scale score <10 and a 17-item Hamilton Depression Rating Scale score <10 for at least 7 days before biopsy. In addition to data from the 17 age- and sex-matched comparison subjects, comparison data were also available for 322 olfactory receptor neurons from 75 healthy subjects previously studied (4).
We performed calcium imaging of enzymatically dissociated olfactory receptor neurons using standard methods and the ratiometric calcium indicator fura-2/AM (3, 5). Cells on coverslips were placed in a recording chamber and continuously superfused with Ringer’s solution (3); stimuli were introduced through the superfusion flow. Mixture A includes citralva, citronellal, eugenol, geraniol, hedione, menthone, and phenylethyl alcohol, and mixture B contains ethyl vanillin, isovaleric acid, lilial, lyral, phenylethylamine, and triethylamine (100 μM each) (3)). Intracellular calcium responses were defined as increase, decrease, or no response as described (4).
Statistical Analysis
Primary dependent measures consisted of basal intracellular calcium and the types of intracellular calcium responses to odorant mixtures expressed as increase or decrease to mixture A or to mixture B. Since multiple cells obtained from each subject are potentially correlated and these data points should be treated as repeated measures, we applied the linear mixed model to account for the within-subject correlation. In doing so, the varied number of cells obtained from each subject was considered random so that the variability in the number of cells from each subject would not affect analyses. For basal intracellular calcium, the model was estimated with the restricted maximum likelihood method by using the SAS procedure PROC MIXED (SAS version 8, SAS Institute, Cary, N.C.). Pairwise comparisons were made through least squares means derived from the model.
To analyze the categorical variables (odorant-induced increases or decreases in intracellular calcium), we adopted a linear mixed model adjusting for the hierarchy of clusters with nested random effects to account for within-subject correlation. This linear mixed model is fit through a pseudo-quasilikelihood approach, implemented with the GLIMMX macro in SAS. The pseudo-quasilikelihood approach uses an iterative process to derive estimates for within-patient and between-patient analyses; therefore, final parameter estimates acquired from the iterative process account for the within-subject correlation. Fisher’s exact test was used for comparison of response frequencies.
Results
Olfactory receptor neurons were clearly identified morphologically; they comprised a cell body, dendrite, and a knob as previously described (3) (Figure 1). Basal intracellular calcium values of olfactory receptor neurons from the four groups (medication-free patients, medicated patients, and age- and sex-matched comparison subjects for each patient group) were compared. The analysis indicated a significant difference among the four groups (F=4.56, df=3, 109, p<0.005). Pairwise comparison showed that basal intracellular calcium values in olfactory receptor neurons (N=30) from seven medication-free patients (mean=46.3 nM, SE=9.9) were significantly lower than the neurons (N=37) of their matched healthy comparison subjects (mean=86.0 nM, SE=13.8) (t=2.21, df=109, p<0.03).
The basal intracellular calcium values in olfactory receptor neurons from the 10 medicated patients (N=37) (mean=29.8 nM, SE=6.2) were significantly lower than those from their matched healthy comparison subjects (N=46) (mean=77.2 nM, SE=7.4) (t=2.64, df=109, p<0.01).
Stimulation of acutely isolated olfactory receptor neurons with mixture A or mixture B resulted in either increases or decreases in intracellular calcium in a subset of olfactory receptor neurons (Figure 1 is an example). No cells responded to both odorant mixtures in this study. Responses of olfactory receptor neurons from healthy comparison subjects were consistent with those seen previously with respect to response type and frequency (Table 1).
Olfactory receptor neurons from medication-free patients exhibited a markedly unusual pattern of responses, responding only to mixture B and only with decreases in intracellular calcium (Table 1). This response frequency was significantly different from that seen in olfactory receptor neurons from their matched healthy comparison subjects (p<0.0001, Fisher’s exact test). Accounting for differences in the number of cells studied from each person, the pseudo-quasilikelihood analysis indicated that a higher proportion of cells from medication-free patients exhibited decreases in intracellular calcium in response to mixture B stimulation compared with those from healthy comparison subjects (F=12.01, df=1, 12, p=0.005).
The response frequency of olfactory receptor neurons from medicated patients was significantly lower than that seen in either the medication-free patients (p=0.001, Fisher’s exact test) or their healthy comparison subjects (p=0.003, Fisher’s exact test) (data not shown). It is noteworthy that the only response type seen in the neurons from medicated patients was a decrease in response to mixture B, as shown in those from medication-free patients.
Discussion
To our knowledge, this is the first attempt to examine neuronal signaling in bipolar disorder using a standard set of stimuli that activate intracellular calcium responses. The odorant mixtures have been extensively used to investigate intracellular signaling in olfactory receptor neurons (3, 6). Our results demonstrate a striking difference in calcium responses between olfactory receptor neurons from medication-free patients and healthy comparison subjects. Olfactory receptor neurons from healthy comparison subjects showed four different response types: increase or decrease in response to either mixture A or mixture B. In contrast, the odorant responsiveness of olfactory receptor neurons from medication-free euthymic patients with bipolar disorder were predominantly decreases in intracellular calcium in response to mixture B (Table 1).
Although the small number of subjects limits the generalizability of the results of this study, the pseudo-quasilikelihood analysis indicates that the proportion of olfactory receptor neurons exhibiting decreases in intracellular calcium in response to mixture B is significantly higher among olfactory receptor neurons from medication-free patients than those from healthy comparison subjects (p=0.005, Fisher’s exact test). A future study with a larger number of subjects is required to assess whether olfactory receptor neurons exhibiting the other types of responses may be present in medication-free patients but simply were not sampled in this study. These data were collected from euthymic medication-free patients and thus may be indicative of a trait of bipolar disorder.
It is intriguing that calcium responses to odorant stimulation in olfactory receptor neurons from medication-free patients are predominantly decreases, which did not appear to be due to a difference in basal calcium levels. The mechanism for this response is currently unknown but may involve removal of calcium by either a sodium/calcium antiporter or calcium ATPase (7) or activation of intracellular calcium uptake into mitochondria or other intracellular stores. An understanding of this process in olfactory receptor neurons from patients with bipolar disorder may provide new targets for the design of therapeutic agents.
In the olfactory receptor neurons from medicated patients, the overall responsiveness to odorants was significantly less than that of olfactory receptor neurons from healthy comparison subjects or medication-free patients. Since odorant-induced intracellular calcium responses are mediated by G-protein-linked effector systems, this finding may reflect attenuation of receptor-mediated G-protein-linked intracellular signaling by mood stabilizer treatment, as shown in other cellular systems (8).
 |
Received March 14, 2003; revision received March 25, 2004; accepted June 10, 2004. From the Department of Psychiatry, University of Pennsylvania, Philadelphia; the University of Scranton, Pa.; the Department of Cellular and Structural Biology, University of Colorado, Denver; the Department of Physiology and Pharmacology, City University of New York Medical School, New York; the Arthur P. Noyes Research Foundation, Norristown, Pa.; the Department of Otolaryngology-Head and Neck Surgery, Thomas Jefferson University, Philadelphia; the Department of Mathematics and Applied Statistics, West Chester University, West Chester, Pa.; and the Monell Chemical Senses Center, Philadelphia. Address correspondence and reprint requests to Dr. Hahn, Department of Psychiatry, University of Pennsylvania, 549 Clinical Research Bldg., 415 Curie Blvd., Philadelphia, PA 19104–6140; [email protected] (e-mail). Funded in part by National Institute on Deafness and Other Communication Disorders grants DC-00214 and DC-02876 (Dr. Rawson) and DC-00244 and DC-00566 (Dr. Restrepo), by NIMH grants MH-60964 (Dr. Friedman) and MH-61912 (Dr. Hahn), and by a grant from the Stanley Foundation (Dr. Hahn). The authors thank Elizabeth Varga and Shay Hyman for expert technical assistance and John Caldwell for discussion.
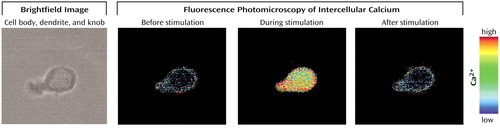
Figure 1. An Example of Intracellular Calcium Responses in Human Olfactory Receptor Neurona
aBrightfield image (40× magnification) shows a human olfactory receptor neuron with the typical round cell body and dendrite with knob. Pseudocolor images reflect intracellular calcium levels before, during, and after stimulation with an odorant mixture (red=maximal calcium, black=0 calcium). This cell responded to stimulation with an increase in intracellular calcium.
1. Dubovsky SL, Christiano J, Daniell LC, Franks RD, Murphy J, Adler L, Baker N, Harris RA: Increased platelet intracellular calcium concentration in patients with bipolar affective disorders. Arch Gen Psychiatry 1989; 46:632–638Crossref, Medline, Google Scholar
2. Dubovsky SL, Murphy J, Christiano J, Lee C: The calcium second messenger system in bipolar disorders: data supporting new research directions. J Neuropsychiatry Clin Neurosci 1992; 4:3–14Crossref, Medline, Google Scholar
3. Rawson NE, Gomez G, Cowart B, Brand JG, Lowry LD, Pribitkin EA, Restrepo D: Selectivity and response characteristics of human olfactory neurons. J Neurophysiol 1997; 77:1606–1613Crossref, Medline, Google Scholar
4. Gomez G, Rawson NE, Cowart B, Lowry LD, Pribitkin EA, Restrepo D: Modulation of odor-induced increases in [Ca(2+)](i) by inhibitors of protein kinases A and C in rat and human olfactory receptor neurons. Neuroscience 2000; 98:181–189Crossref, Medline, Google Scholar
5. Lowry LD, Pribitkin EA: Collection of human olfactory tissue, in Experimental Biology of Taste and Olfaction. Edited by Spielman AI, Brand JG. Boca Raton, Fla, CRC Press, 1995, pp 47–48Google Scholar
6. Breer H, Boekhoff I, Krieger J, Raming K, Strotmann J, Tareilus E: Molecular mechanisms of olfactory signal transduction. Soc Gen Physiol Ser 1992; 47:93–108Medline, Google Scholar
7. Noe J, Tareilus E, Boekhoff I, Breer H: Sodium/calcium exchanger in rat olfactory neurons. Neurochem Int 1997; 30:523–531Crossref, Medline, Google Scholar
8. Lenox RH, Hahn CG: Overview of the mechanism of action of lithium in the brain: fifty-year update. J Clin Psychiatry 2000; 61(suppl 9):5–15Google Scholar



