Low Gene Expression Conferred by Association of an Allele of the 5-HT2C Receptor Gene With Antipsychotic-Induced Weight Gain
Abstract
OBJECTIVE: Association has been reported between the C allele of a –759C/T polymorphism in the promoter of the 5-HT2C receptor gene (HTR2C) and antipsychotic-induced weight gain, suggesting that polymorphic HTR2C expression influences this phenotype. The authors tested this polymorphism, and other promoter variants, for effects on HTR2C transcription. METHOD: Six HTR2C promoter haplotypes constructed from four polymorphisms were cloned into a luciferase reporter gene plasmid. Their transcriptional activities were then compared in two human cell lines. RESULTS: All haplotypes containing the –759C allele showed less transcriptional activity than haplotypes containing the –759T allele. The A allele of a –997G/A polymorphism was also associated with reduced expression. CONCLUSIONS: These findings suggest that the –759C allele is functional and results in relative underexpression of HTR2C. Reduced expression of HTR2C mRNA may underlie vulnerability to weight gain following antipsychotic treatment.
Weight gain is a common adverse reaction to treatment with antipsychotic drugs (1). Clinically, this is a particular problem with many atypical antipsychotic medications. Rather than the movement disorders that plagued the use of typical antipsychotics, weight gain is a major reason for discontinuation or noncompliance of the atypical antipsychotics. It has been proposed that a serotonergic mechanism underlies antipsychotic-induced weight gain and that the problem is particularly associated with antipsychotics with relatively high affinity for serotonin (5-HT) receptors (1). Compatible with this hypothesis, two studies (2, 3) have suggested that antipsychotic-induced weight gain is associated with the C allele of a –759C/T polymorphism of the HTR2C gene encoding the 5-HT2C receptor.
Since the HTR2C –759C/T polymorphism does not appear to be in the primary transcript and cannot affect mRNA stability, splicing, editing, or the structure or function of the encoded receptor, it follows that for the association to be a causative one, it must be associated with effects on HTR2C transcription. The polymorphism is 31 base-pairs upstream of the putative start of HTR2C transcription and is therefore likely to be in the core promoter of the gene to which the RNA polymerase II complex binds. However, the location of the promoter has not been confirmed experimentally. In this study, we sought to confirm that the region containing this polymorphism is a true promoter. We also studied its effect and the effect of other promoter polymorphisms on gene transcription using a reporter gene assay.
Method
Primers were designed to amplify 500 base-pairs of the 5′ flanking sequence of HTR2C based on National Center for Biotechnology Information reference sequence NM_000868 and NT_028405.9. Primers used were 5′ GTTTGACCCTGTGAGTGCCT (forward) and 5′ GCACCAGAGCGCCTACCTC (reverse). Haplotypes representing all known polymorphisms were identified by sequencing genomic DNA from a panel of eight anonymous subjects. Approximate minor allele frequencies were estimated in pooled DNA from 100 local blood donors by automated sequencing.
Details of the cloning procedure have been described in detail previously (4). Briefly, purified polymerase chain reaction (PCR) products were ligated into a modified pGL3 luciferase expression vector and clones with the required insert selected by PCR, sequenced to confirm fidelity, and used to transfect eukaryotic cells in culture.
The ability of each sequence to promote transcription of the luciferase gene was tested transiently in human cell lines HEK293t (human embryo kidney, a gift from GlaxoSmithKline) and TE671 (human medulloblastoma/rhabdomyosarcoma from the European Collection of Cell Cultures [ECACC]) as previously described (4).
Cell lines were transfected with plasmid by using lipofectamine (Gibco BRL, Gaithersburg, Md.) in 96-well format (eight replicates per clone for each cell line) and cultured according to ECACC specifications at 37°C with 5% CO2 in 96-well luminometric plates. The assays were repeated three times with fresh plasmid preparation to discount the possibility of differences being attributable to random variation in the plasmid preparation procedure.
To control for transfection efficiency, cells were cotransfected with CMV-SEAP (an expression plasmid containing the secreted placental alkaline phosphatase [SEAP] gene under the control of the cytomegalovirus [CMV] promoter; a gift from GlaxoSmithKline). Promoter activity was normalized by dividing luciferase activity by SEAP activity.
Results
Four polymorphisms—three single nucleotide polymorphisms (SNPs) and a variable length GT repeat—were found in the putative promoter region of the HTR2C gene. Each was previously known and listed in dbSNP (http://www.ncbi.nlm.nih.gov/SNP/index.html) as rs3813929 (–759C∼90%/T∼10%—relative to the start of translation), rs3813928 (–997G∼90%/A∼10%), rs3834996 (–1028GT12∼30%/GT15/16∼70%), and rs498207 (–1165A∼90%/G∼10%). No other SNPs are listed in dbSNP. We give the positions of the polymorphisms based on the genomic reference sequence NT_028405.9, using the start of translation as given by mRNA reference sequence NM_000868.
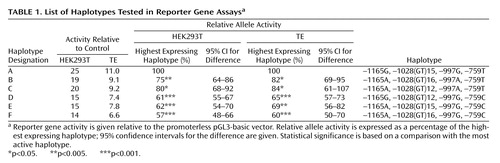
Six different haplotypes were found containing the four polymorphisms. These are designated as A to F to aid discussion (Table 1). Reporter gene constructs in both HEK and TE cell lines gave qualitatively similar results. Taken together with their location relative to the putative transcription start site, the high levels of expression relative to the negative control suggest it is highly likely that the constructs contain the core promoter (4).
A comparison of haplotypes B and C, which differ only by the length of the –1028GT repeat, shows that a difference of four GT repeats makes little difference to the activity of the promoter. Similarly, a comparison of haplotypes E and F shows that a difference of one GT repeat also has little effect. A comparison of haplotype D with either E or F shows that the –1165G/A polymorphism has no effect. The three haplotypes with –759T (A, B, and C) had higher transcriptional activity than those with a –759C (D, E, and F) regardless of sequence at the other sites. Moreover, a comparison of haplotypes A and D, which differ only by the nonfunctional three GT repeats and the –759C/T, shows that the –759C allele decreases transcription to approximately 60% of the –759T allele in both cell lines. Assuming no interactive effects with either the –1165 or –1028 sites, comparison of haplotype A with both haplotypes B and C shows that allele –997A lowers the activity by around 20% compared with –997G.
Discussion
The propensity for antipsychotic medications, particularly the atypical antipsychotics, to cause weight gain in patients is well-known, although the underlying cause of these effects is not known (1). The role of the 5-HT2C receptor in appetite control is also well-known (5). Recently, genetic evidence that sequence variation in this gene is relevant to weight-related phenotypic variation has been provided by two studies showing that the C allele of a common C/T polymorphism in the promoter region of the 5-HT2C receptor is associated with antipsychotic-induced weight gain (2, 3). However, as is typical for alleles of modest effect, support is not universal (6).
Yuan et al. (5) reported differences in transcriptional activity between 5-HT2C promoter haplotypes, but they were unable to discriminate between the effects of each individual polymorphism. Therefore, to test the functional plausibility of this association, we studied the –759C/T and other 5-HT2C receptor promoter region polymorphisms in a luciferase reporter gene assay. We made reporter gene clones of six haplotypes representing four polymorphisms in the first 500 base-pairs upstream of the start of transcription of HTR2C, allowing us to assess the effect of each. Our data suggest that the –1165G/A and the –1028GT dinucleotide repeat number have little if any independent effect on transcription. In comparison, the –997G/A has a small effect, and the –759C/T has a relatively large effect. In the case of –997G/A, the more common G allele gives the higher activity, but in the case of –759C/T, the less common –759T allele gives the higher activity. The results from both cell lines were remarkably similar and consistent with the results of Yuan et al. (5), who used P19 embryonal carcinoma cells. This suggests that unique tissue-specific factors are not involved in the effects we have seen and the effects are likely to occur in any tissue where the 5-HT2C receptor is expressed.
Our finding that the associated polymorphism is functional considerably enhances the plausibility that the previous reports represent true associations. Taken with those studies, our data suggest that higher basal expression as a result of the HTR2C –759T allele is protective against antipsychotic-induced weight gain or, conversely, that lower basal expression represents vulnerability. In animal studies, we have previously shown that several antipsychotics, including clozapine, down-regulate HTR2C mRNA expression in rat brain following chronic treatment (7). Although to our knowledge this effect has not been examined in patients, we propose that high basal HTR2C may act to minimize this effect, and that this is at least one of the mechanisms underpinning some of the variance in the weight-gain response to antipsychotics. Of relevance to future genetic studies, our data also predict that haplotypes constructed from the –997G/A and –759C/T polymorphisms may provide a more powerful prediction of weight gain than either marker alone.
 |
Received Dec. 10, 2003; revision received Feb. 24, 2004; accepted April 23, 2004. From the Department of Psychological Medicine, University of Wales College of Medicine. Address correspondence and reprint requests to Dr. Buckland, Department of Psychological Medicine, University of Wales College of Medicine, Heath Park, Cardiff, CF14 4XN, U.K.; [email protected] (e-mail). Funded by U.K. Medical Research Council grant G9814784.
1. Nasrallah H: A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology 2003; 28:83–96Medline, Google Scholar
2. Reynolds GP, Zhang ZJ, Zhang XB: Association of antipsychotic drug-induced weight gain with a 5-HT2C receptor gene polymorphism. Lancet 2002; 359:2086–2087Crossref, Medline, Google Scholar
3. Reynolds GP, Zhang ZJ, Zhang XB: Polymorphism of the promoter region of the serotonin 5-HT2C receptor gene and clozapine-induced weight gain. Am J Psychiatry 2003; 160:677–679Link, Google Scholar
4. Coleman SL, Buckland PR, Hoogendoorn B, Guy C, Smith SK, O’Donovan MC: Experimental analysis of the annotation of promoters in the public database. Hum Mol Genet 2002; 11:1817–1821Crossref, Medline, Google Scholar
5. Yuan X, Yamada K, Ishiyama-Shigemoto S, Koyama W, Nonaka K: Identification of polymorphic loci in the promoter region of the serotonin 5-HT2C receptor gene and their association with obesity and type II diabetes. Diabetologia 2000; 43:373–376Crossref, Medline, Google Scholar
6. Tsai SJ, Hong CJ, Yu YW, Lin CH: -759C/T genetic variation of 5HT(2C) receptor and clozapine-induced weight gain (letter). Lancet 2002; 360:1790Crossref, Medline, Google Scholar
7. Buckland PR, D’Souza U, Maher NA, McGuffin P: The effects of antipsychotic drugs on the mRNA levels of serotonin 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res 1997; 48:45–52Crossref, Medline, Google Scholar



