Association of a Functional Polymorphism in the Serotonin Transporter Gene With Abnormal Emotional Processing in Ecstasy Users
Abstract
OBJECTIVE: The long-term effects of the use of 3,4-methylenedioxymethamphetamine (MDMA, or Ecstasy) in humans are controversial and unclear. The authors’ goal was to assess the contribution of a functional polymorphism in the gene encoding serotonin transporter to changes in emotional processing following chronic Ecstasy use. METHOD: They investigated Beck Depression Inventory scores and performance on the Affective Go/No-Go test, a computerized neuropsychological test sensitive to emotional processing, in Ecstasy users and comparison subjects, stratifying the results by serotonin transporter genotype. RESULTS: Ecstasy use was associated with higher Beck Depression Inventory score and abnormalities in the Affective Go/No-Go test in individuals with the ss and ls genotype but not those with the ll genotype. CONCLUSIONS: Ecstasy users carrying the s allele, but not comparison subjects carrying the s allele, showed abnormal emotional processing. On the basis of a comparison with acute tryptophan depletion, the authors hypothesize that chronic Ecstasy use may cause long-term changes to the serotonin system, and that Ecstasy users carrying the s allele may be at particular risk for emotional dysfunction.
An estimated 500,000–2,000,000 tablets of 3,4-methylenedioxymethamphetamine (MDMA, or Ecstasy) are taken recreationally each week in England (1). MDMA binds to the serotonin transporter (5-HTT), preventing reuptake and stimulating release of serotonin (5-HT), and causes long-term changes to the 5-HT system in rats, nonhuman primates, and humans (2–4). In view of the critical role of 5-HT in the regulation of mood and the importance of selective serotonin reuptake inhibitors (SSRIs) in the treatment of major depression, it has been suggested that Ecstasy users may be at greater risk for developing affective disturbance following chronic use (5). However, the long-term psychiatric consequences of Ecstasy use are highly controversial (6).
The gene coding for the 5-HTT contains a functional polymorphism in the promoter region, a 44-base-pair insertion/deletion approximately 1 kilobase upstream of the transcription initiator site, designated the 5-HTT gene-linked polymorphic region (5-HTTLPR). This polymorphism produces two alleles, designated l (“long”) and s (“short”), respectively (7). Cells with the l allele have been shown to express more 5-HTT than cells with the s allele, and, concordant with this, reuptake of 5-HT in human lymphoblastoid cells homozygous for the l allele has been shown to be approximately twice that of cells either heterozygous or homozygous for the s allele (7).
We investigated Beck Depression Inventory scores and performance on the Affective Go/No-Go test, which is sensitive to emotional processing, in Ecstasy users and comparison subjects. We stratified individuals by 5-HTTLPR genotype, since the 5-HTT is the primary site of action for MDMA and individuals with the s allele are at greater risk for affective disorders (8) and also show poor response to antidepressant treatment with SSRIs (9). We hypothesized that the 5-HTTLPR s allele would confer particular risk for emotionally related cognitive disturbance following Ecstasy use because this allele is associated with lower 5-HTT expression (8) and because experimental animal models of Ecstasy use show increased anxiety and decreased 5-HTT levels (2).
Method
Sixty-six Ecstasy users (48 men, mean age=24.2 years, SD=6.6, mean IQ=112, SD=6.9), 30 cannabis users (15 men, mean age=25.7, SD=8.9, mean IQ=112, SD=8.8) and 28 healthy volunteers with no history of illicit drug use (15 men, mean age=24.0, SD=3.7, mean IQ=114, SD=5.4) were recruited by advertisement from the community. The Ecstasy users had to have used Ecstasy on at least 30 separate occasions and were required to abstain from use for at least 3 weeks before testing. Neither the cannabis users nor the healthy comparison subjects had used Ecstasy. Premorbid IQ was estimated by using the National Adult Reading Test (10). In addition, a 10-ml blood sample was taken, and all participants filled out a structured substance use questionnaire and the Adult Impulsiveness, Venturesomeness and Empathy Scale (11), a widely used 54-item personality questionnaire producing three orthogonal constructs that relate in a factor analysis to impulsiveness (self-control), venturesomeness (risk-taking/sensation seeking), and empathy.
Participants reporting any drug use on the day of testing were excluded, as were any showing a positive plasma screen for MDMA/amphetamines (analysis carried out by enzyme assay followed by high-performance liquid chromatography/mass spectroscopy [Tricho-Tech, Cardiff, U.K.—www.tricho-tech.co.uk]). Genomic DNA was extracted from blood by using standard methods, and the 5-HTTLPR was determined by polymerase chain reaction followed by size separation of product (8). All subjects provided written informed consent, and the study was approved by the Cambridge Local Research Ethics Committee.
Affective disturbance was examined by using the Beck Depression Inventory (12) and the Affective Go/No-Go test (13). The Beck Depression Inventory is a well-validated, 21-item, self-report rating scale for depression. The questions cover a variety of different symptoms of depression, and for each a score from 0 (no symptom) to 3 (severe symptom) can be recorded. Thus the maximum score is 63, although scores higher than 40 are rarely recorded. The Beck Depression Inventory manual recommends a cutoff point of 9 for mild depression.
The Affective Go/No-Go test is a computerized neuropsychological test, details of which have been described previously (13). On each trial, a word that is either happy or sad appears briefly on the screen, and subjects must make or withhold a response on the basis of the emotion of the word within approximately 1 second. On each block of 18 trials, half the words are happy and half are sad, and subjects are instructed that they must respond either to the happy words or to the sad words. Every two blocks, the targets and the distractors change—words that were previously targets become distractors and vice versa. Such blocks are defined as “shift” blocks, and blocks where the targets and distractors are of the same emotion as in the previous block are defined as “nonshift.” Three measures are recorded for each block: mean correct response latency, total false alarms (commission errors), and total misses (omission errors).
In the Affective Go/No-Go test, healthy volunteers typically respond faster to happy words than sad words, depressed patients show the opposite pattern of results, and both groups reduce commission errors from shift to nonshift blocks (13). The test is sensitive to monoamine depletions (14–16), and a neuroimaging version has been developed in which patients with depression show enhanced neural response to sad distractors (17).
Results
The drug-naive comparison group and the cannabis-using comparison group did not differ on any behavioral measure, either overall or when genetic subgroups were compared directly, and for the purpose of analysis were pooled into a single comparison group. The genotype frequencies in the comparison group (ll N=21 [36%], ls N=26 [45%], ss N=11 [19%]) and the Ecstasy users (ll N=20 [30%], ls N=31 [47%], ss N=15 [23%]) did not differ from the values expected under Hardy-Weinberg assumptions (χ2=0.20, df=1, p=0.65, and χ2=0.34, df=1, p=0.56, respectively). There were no significant differences in 5-HTTLPR genotype frequency (χ2=0.56, df=2, p=0.75) and allele frequency (χ2=0.59, df=2, p=0.44) in the Ecstasy and comparison groups, suggesting that the 5-HTTLPR is not a major predictor of Ecstasy use. The genetic subgroups did not differ in terms of age, IQ, or any measure of illicit drug use in either the Ecstasy users or the comparison subjects.
Repeated-measures analysis of variance (ANOVA) was used to examine data from the Affective Go/No-Go test. There was a highly significant shift-by-genetic-subgroup interaction for commission errors in the Ecstasy users (F=6.81, df=2, 64, p<0.003) but not in the comparison subjects (F=0.16, df=2, 55, p=0.85). In the ll subgroup of the Ecstasy users, and in all genetic subgroups in the comparison group, commission errors declined as expected from shift to nonshift blocks. However, the ls and ss subgroups of Ecstasy users did not make the expected reduction in errors from shift to nonshift blocks (Figure 1).
Since all Ecstasy users in this study also took other drugs, we conducted further analyses to control for the possible effects of non-Ecstasy illicit drug use. The ANOVA was repeated within the Ecstasy users, including non-Ecstasy illicit substance use (classed as either regular or rarely/never) as an additional between-subjects factor in separate analyses for psilocybin, LSD, amphetamine, amyl nitrate, ketamine, cocaine, and heroin. In no instance was the interaction term between drug use group, genotype group, and shift significant. However, because almost all Ecstasy users also used cannabis on a regular basis, this method was not appropriate to control for cannabis use. Instead, we conducted the analysis in the cannabis comparison subjects alone and did not find an interaction between genetic subgroup and shift.
Depression scores were not normally distributed and were analyzed in two ways. First, the Mann-Whitney U test was used to compare Beck Depression Inventory scores of the Ecstasy users and comparison subjects within each genetic subgroup. The Ecstasy users scored significantly higher on the Beck Depression Inventory than comparison subjects within the ss (Ecstasy group mean=11.8, SD=9.6; comparison group mean=3.9, SD=3.2) (z=2.4, p<0.02) and ls (Ecstasy group mean=8.1, SD=5.5; comparison group mean=5.2, SD=5.6) (z=2.4, p<0.02) genotype subgroups, but the difference in the ll subgroup was not significant (Ecstasy group mean=7.5, SD=6.0; comparison group mean=5.3, SD=3.1) (z=1.0, p=0.32).
Second, each individual was categorized as either not depressed or depressed on the basis of the accepted cutoff point of 9 on the Beck Depression Inventory. The proportions of individuals categorized as depressed or not depressed showed a strong tendency to differ in the Ecstasy users when classified by 5-HTTLPR genotype (χ2=5.95, df=2, p<0.06)—there were more individuals categorized as depressed in the ss subgroup of Ecstasy users. The proportions of depressed versus nondepressed individuals did not show this tendency to differ as a function of 5-HTTLPR genotype in the comparison group (χ2=0.55, df=2, p=0.76).
Adult Impulsiveness, Venturesomeness and Empathy Scale scores were analyzed in a univariate ANOVA with group and genetic subgroup as between-subjects measures. The Ecstasy users scored higher than the comparison subjects on impulsiveness (F=5.8, df=1, 118, p<0.02), with no effect of genetic subgroup and no group-by-genetic-subgroup interaction (F<1, df=2, 118, for both). However, amphetamine exposure correlated highly significantly with impulsiveness in the Ecstasy users (F=15.2, df=1, 56, p<0.001), and when those who had used amphetamine regularly were removed from the analysis, the remaining Ecstasy users (N=40) were not significantly more impulsive than the comparison subjects (F<1, df=1, 96). There were no differences between the Ecstasy and non-Ecstasy groups or genetic subgroups, and there were no interaction effects on venturesomeness or empathy (p>0.1 for all).
Discussion
We believe that these are the first data to suggest that the 5-HTTLPR genotype mediates emotionally related cognitive disturbance in Ecstasy users. Because Ecstasy causes long-term reductions in synaptic 5-HT release (18), it is of interest to note the similarities of our current results to those seen in normal subjects after acute tryptophan depletion, a dietary manipulation that lowers availability of the precursor of 5-HT to the brain and temporarily reduces synthesis (19). Healthy volunteers under conditions of acute tryptophan depletion show the same behavior on the Affective Go/No-Go test as the ls and ss groups of Ecstasy users—they fail to reduce commission errors from shift to nonshift blocks (15). Moreover, ss individuals who have never suffered from depression show the greatest mood change under acute tryptophan depletion, while ll individuals show little or no mood change (20), a result concordant with our finding that ss Ecstasy users were the group that scored highest on the Beck Depression Inventory. It is possible that possession of the s allele confers particular vulnerability to disturbances in emotional processing following 5-HT depletion, whether by acute tryptophan depletion or chronic Ecstasy use, perhaps due to low levels of tonic serotonergic neurotransmission.
This similarity with acute tryptophan depletion is intriguing, but it remains possible that the differences between the genetic subgroups among the Ecstasy users are caused by some other factor. Data from the Adult Impulsiveness, Venturesomeness and Empathy Scale did not provide evidence of personality differences between the genetic subgroups. In fact, our results suggest that the greater impulsivity in Ecstasy users found in other reports may be due to concomitant use of amphetamine. However, Soar et al. (21) calculated that 34% of those in whom Ecstasy triggers a psychiatric disorder had a family history of psychopathology, and it may be that Ecstasy users carrying the s allele have a family history of depression.
In summary, we have identified a test of emotional processing that is overtly abnormal in chronic Ecstasy users with specific 5-HTTLPR genotypes carrying the s allele. These data are compatible with the finding of a trend toward higher depressive scores on the Beck Depression Inventory in Ecstasy users with the ss genotype. Since such effects may have been overlooked if we had not stratified our samples by this genotype, future studies examining the long-term effects of Ecstasy use should consider the potential for gene-environment interactions at the 5-HTTLPR locus.
Received Dec. 2, 2003; revision received March 23, 2004; accepted May 27, 2004. From the Department of Psychiatry, University of Cambridge School of Clinical Medicine, and the Department of Medical Genetics, Cambridge Institute for Medical Research, Addenbrooke’s Hospital. Address correspondence and reprint requests to Professor Sahakian, Department of Psychiatry, University of Cambridge School of Clinical Medicine, Addenbrooke’s Hospital, Cambridge CB2 2QQ, U.K.; [email protected] (e-mail). Funded by Wellcome Trust Program grant 019407 (Trevor Robbins, Professor Sahakian, Barry Everitt, and Angela Roberts). Dr. Rubinsztein is a Wellcome Trust Senior Fellow in Clinical Science, and Mr. Roiser was funded by a Medical Research Council Studentship. The authors thank Caroline Humphries and other staff members of the Wellcome Trust Clinical Research Facility for their help and support, all the volunteers who took part in the study, the Cambridge Evening News for their assistance in recruitment, and Trevor Robbins and Andrew Blackwell for valuable discussion of the manuscript.Professor Sahakian is a consultant for Cambridge Cognition.
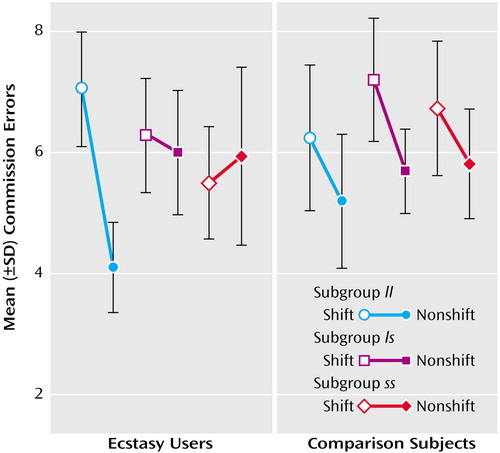
Figure 1. Commission Errors on the Affective Go/No-Go Test of 66 Ecstasy Users and 58 Comparison Subjects, Stratified by Genotypea
aCommission errors are on shift blocks compared with nonshift blocks on the Affective Go/No-Go test. Among the Ecstasy users, 20 subjects had the ll subtype, 31 had the ls subtype, and 15 had the ss subtype. Among the comparison subjects (30 cannabis users and 28 healthy subjects with no history of drug use), 21 had the ll subtype, 26 had the ls subtype, and 11 had the ss subtype. In comparison subjects, each genetic subgroup shows the expected reduction from shift to nonshift blocks. However, in the Ecstasy users, only the ll subgroup shows the expected reduction from shift to nonshift blocks, while the ls and ss subgroups show no difference in error rates between shift and nonshift blocks. Error bars represent one standard deviation.
1. Ecstasy and Amphetamines: Global Survey 2003. Vienna, United Nations Office on Drugs and Crime, 2003Google Scholar
2. Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI: The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 2003; 55:463–508Crossref, Medline, Google Scholar
3. Reneman L, Booij J, de Bruin K, Reitsma JB, de Wolff FA, Gunning WB, den Heeten GJ, van den Brink W: Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet 2001; 358:1864–1869Crossref, Medline, Google Scholar
4. Kish SJ, Furukawa Y, Ang L, Vorce SP, Kalasinsky KS: Striatal serotonin is depleted in brain of a human MDMA (Ecstasy) user. Neurology 2000; 55:294–296Crossref, Medline, Google Scholar
5. MacInnes N, Handley SL, Harding GF: Former chronic methylenedioxymethamphetamine (MDMA or ecstasy) users report mild depressive symptoms. J Psychopharmacol 2001; 15:181–186Crossref, Medline, Google Scholar
6. Cole JC, Sumnall HR: The BDI of the beholder (letter). J Psychopharmacol 2002; 16:103Crossref, Medline, Google Scholar
7. Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL: Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531Crossref, Medline, Google Scholar
8. Furlong RA, Ho L, Walsh C, Rubinsztein JS, Jain S, Paykel ES, Easton DF, Rubinsztein DC: Analysis and meta-analysis of two serotonin transporter gene polymorphisms in bipolar and unipolar affective disorders. Am J Med Genet 1998; 81:58–63Crossref, Medline, Google Scholar
9. Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M: Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry 1998; 3:508–511Crossref, Medline, Google Scholar
10. Nelson HE: National Adult Reading Test (NART): Test Manual. Windsor, UK, National Foundation for Educational Research-Nelson, 1982Google Scholar
11. Eysenck HJ, Eysenck SBG: Adult Impulsiveness, Venturesomeness and Empathy Scale. London, Hodder and Stoughton, 1991Google Scholar
12. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
13. Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES: Emotional bias and inhibitory control processes in mania and depression. Psychol Med 1999; 29:1307–1321Crossref, Medline, Google Scholar
14. Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ: The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002; 163:42–53Crossref, Medline, Google Scholar
15. Rubinsztein JS, Rogers RD, Riedel WJ, Mehta MA, Robbins TW, Sahakian BJ: Acute dietary tryptophan depletion impairs maintenance of “affective set” and delayed visual recognition in healthy volunteers. Psychopharmacology (Berl) 2001; 154:319–326Crossref, Medline, Google Scholar
16. McLean A, Rubinsztein JS, Robbins TW, Sahakian BJ: The effects of tyrosine depletion in normal healthy volunteers: implications for unipolar depression. Psychopharmacology (Berl) 2004; 171:286–297Crossref, Medline, Google Scholar
17. Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ: The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry 2002; 59:597–604Crossref, Medline, Google Scholar
18. Matuszewich L, Filon ME, Finn DA, Yamamoto BK: Altered forebrain neurotransmitter responses to immobilization stress following 3,4-methylenedioxymethamphetamine. Neuroscience 2002; 110:41–48Crossref, Medline, Google Scholar
19. Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PD, Price LH, Heninger GR, McDougle CJ: Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology 1998; 19:26–35Crossref, Medline, Google Scholar
20. Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, Praschak-Rieder N, Zach J, de Zwann M, Bondy B, Ackenheil M, Kasper S: Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry 2002; 59:613–620Crossref, Medline, Google Scholar
21. Soar K, Turner JJ, Parrott AC: Psychiatric disorders in Ecstasy (MDMA) users: a literature review focusing on personal predisposition and drug history. Hum Psychopharmacol 2001; 16:641–645Crossref, Medline, Google Scholar



