Association Between Reduced Extraversion and Right Posterior Fusiform Gyrus Gray Matter Reduction in Chronic Schizophrenia
Abstract
OBJECTIVE: The authors examined the association between volume of the fusiform gyrus, a region involved in face processing, and the personality trait of extraversion in patients with schizophrenia. METHOD: Male patients (N=24) and age-matched male comparison subjects (N=26) completed NEO Five-Factor Inventory personality measures of extraversion and underwent high-spatial-resolution magnetic resonance imaging of anterior and posterior fusiform gyrus gray matter. RESULTS: Low extraversion scores were significantly correlated with gray matter volume reductions in the right posterior fusiform gyrus for patients but not comparison subjects. CONCLUSIONS: Reduced right posterior fusiform gyrus volume may contribute to disease-related social disturbances, characterized by both low extraversion and reduced sensitivity to human faces.
Patients with schizophrenia often fail to recognize previously seen faces, and these difficulties may represent a specific deficit independent of the well-known disease-related generalized impairment of memory and visual attention (1). Face perception is mediated by a well-demarcated, distributed hierarchical neural system, the core of which consists of bilateral occipitotemporal regions in the extrastriate visual cortex, most notably the fusiform gyrus (2). Postmortem (3) and manual region of interest studies in patients with chronic (4) and first-episode (5) schizophrenia have all demonstrated fusiform gyrus abnormalities in schizophrenia patients. These in turn have correlated with lower scores on a neuropsychological test of delayed facial recognition memory in a sample of chronic patients (4).
The highly evolved face recognition ability of humans represents an important component of species social communication, naturally selected to solve adaptive problems critical for survival, such as distinguishing friend from foe, familiar from unfamiliar, related from unrelated. Schizophrenia is invariably accompanied by disturbances in social communication that may even predate disease onset (6). Unaffected biological relatives of patients also show reduced facial recognition memory, which is correlated with schizotypal personality traits (1). In a group of patients with chronic schizophrenia, we therefore explored the relationship between magnetic resonance imaging (MRI) volumes of the fusiform gyrus and sociality, as measured by the highly hereditable personality trait of extraversion (7).
Method
Twenty-four male medicated patients with chronic schizophrenia and 26 age- and parental socioeconomic status-matched male comparison subjects completed the NEO Five-Factor Inventory, Form S (8), a self-report measure of five personality traits: neuroticism, extraversion, openness, agreeableness, and conscientiousness. Subject recruitment, inclusion criteria, and diagnostic evaluations followed our previous publications (e.g., reference 4). The mean age of the patients was 41.8 years (SD=8.1), their mean age at symptom onset was 23.2 years (SD=4.4), and their mean duration of illness was 18.6 years (SD=9.0). The mean medication dose in chlorpromazine equivalents was 495.4 mg/day (SD=259) (nine of the 24 patients were receiving typical antipsychotics, 14 were receiving atypical antipsychotics, and one was receiving both). The mean age of the comparison group was 43.0 years (SD=7.1).
MR images were acquired with a 1.5-Tesla General Electric scanner (GE Medical Systems, Milwaukee) at the Brigham and Women’s Hospital in Boston. The protocol followed that of our previous publication (4). Manual drawings of the fusiform gyrus were performed on the coronal plane, blind to diagnosis. Briefly, the anterior landmark was reliably defined by one slice posterior to mamillary body. The posterior landmark was determined by the anterior tip of the parieto-occipital sulcus in the midsagittal plane. The last slice, which included the crux of the fornix, provided the boundary for subdivision of the fusiform gyrus into anterior and posterior. The collateral sulcus was used as the medial border. The occipitotemporal sulcus was used to determine the lateral border. Figure 1 shows the regions of interest of the fusiform gyrus in axial, sagittal, and coronal slices as well as a three-dimensional reconstruction of the fusiform gyrus. Interrater reliability was computed for the regions of interest by three independent raters, who were blind to group membership. High intraclass correlations were seen for the left anterior (0.94), right anterior (0.94), left posterior (0.95), and right posterior (0.95) fusiform gyrus.
A near-significant group difference in total intracranial content was revealed (t=1.81, df=48, p=0.08). We therefore used relative fusiform gyrus volumes ([absolute fusiform gyrus volume/intracranial content] × 100) for region of interest analysis. For region of interest analysis the standardized scores were submitted to a mixed-model, repeated-measures analysis of variance (ANOVA) with group (schizophrenia, comparison) as a between-subject factor and hemisphere (left, right) and subdivision (anterior, posterior) as within-subject factors. For correlations between regions of interest and T scores for extraversion, Spearman’s correlation was used to diminish the effect of any outliers. In this analysis we used p≤0.0125 as the cutoff value for statistical significance for fusiform gyrus subregions (four regions) and extraversion scores. In addition, simple linear regression analysis was performed to predict T scores for extraversion with fusiform gyrus gray matter relative volumes as independent values for each group.
Results
Overall NEO scores revealed significant group (F=7.93, df=5, 44, p<0.001) and group-by-scale effects (F=9.29, df=4, 45, p<0.001). In relation to the comparison group the patients showed a distinct NEO profile of increased neuroticism (F=38.59, df=1, 48, p<0.001) but reduced extraversion (F=9.36, df=1, 48, p=0.004) and conscientiousness (F=18.76, df=1, 48, p<0.001). ANOVA of standardized region of interest values (z scores) revealed a significant main effect of group (F=6.96, df=1, 48, p=0.01). For the fusiform gyrus, schizophrenia patients had bilateral reductions in anterior and posterior fusiform gyrus gray matter volumes relative to comparison subjects (left anterior: mean=2.54 [SD=0.44] versus 3.04 [SD=0.59], respectively; left posterior: mean=3.15 [SD=0.66] versus 3.66 [SD=0.81]; right anterior: mean=2.56 [SD=0.51] versus 2.93 [SD=0.54]; right posterior: mean=3.40 [SD=0.91] versus 3.77 [SD=0.74]).
Table 1 shows Spearman’s rho correlations between relative fusiform gyrus volumes and T scores for extraversion in both groups. For patients but not comparison subjects, right posterior fusiform gyrus gray matter volume correlated significantly with extraversion score (rho=0.57, p=0.004 [absolute]; rho=0.52, p=0.009 [relative]). Fisher’s z transformation revealed a significant effect for the right posterior fusiform gyrus/extraversion correlation for the patient group (z=–2.23, p=0.01 [absolute]; z=–2.27, p=0.01 [relative]). A simple linear regression analysis revealed a relationship between extraversion score and right posterior fusiform gyrus volume (β=139.8, SE=62.7; t=2.23, p<0.04) but not left posterior (β=–86.5, SE=88.4), right anterior (β=–1.7, SE=139.8), or left anterior (β=–43.9, SE=148.0) volume (all p>0.34). Finally, age, socioeconomic status, parental socioeconomic status, WAIS-R information subscale scores, duration of illness, and chlorpromazine-equivalent medication dose did not correlate significantly with fusiform gyrus volumes.
Discussion
Patients with schizophrenia showed a distinct NEO personality profile of increased neuroticism and decreased conscientiousness and extraversion. Reduced extraversion, in turn, correlated with abnormalities of the face-sensitive right posterior fusiform gyrus. We previously demonstrated a significant correlation between these same fusiform gyrus abnormalities and reduced facial memory recognition (4). Thus, in schizophrenia, reductions in both extraversion and facial memory correlate with smaller right posterior fusiform gyrus volumes. These empirical relationships may eventually help to elucidate the neurobiology of the social disturbance of schizophrenia. However, future studies will need to adopt a multiple-prong approach. Personality measures, symptom ratings, and neuropsychological test scores along with brain imaging will likely be necessary to capture the dynamic multivariate relationship of brain and social personality changes in schizophrenia.
 |
Received Nov. 12, 2003; revision received June 14, 2004; accepted June 30, 2004. From Harvard Medical School’s VA Boston Healthcare System–Brockton Division (Department of Psychiatry, Laboratory of Neuroscience, Clinical Neuroscience Division) and Brigham and Women’s Hospital (Department of Radiology, Surgical Planning Laboratory, MRI Division), Boston. Address correspondence and reprint requests to Dr. McCarley or Dr. Shenton, Department of Psychiatry (116A), Boston VA Healthcare System–Brockton Division, Harvard Medical School, 940 Belmont St., Brockton, MA 02301; [email protected]; [email protected] (e-mail). Supported in part by Department of Veterans Affairs Merit Awards to Drs. Niznikiewicz, Shenton, Nestor, and McCarley; a Middleton Award from the Department of Veterans Affairs to Dr. McCarley; NIMH grants to Dr. Shenton (K02 MH-01110 and R01 MH-50747) and Dr. McCarley (R01 MH-40799); and a grant from the Welfide Medicinal Research Foundation, Japan (Dr. Onitsuka). The authors thank Marie M. Fairbanks for administrative support and Sarah K. Toner, B.A., Lisa C. Lucia, B.S., Meredith C. Klump, B.A., and Sarah M. Rabbitt, B.A., for research assistance.
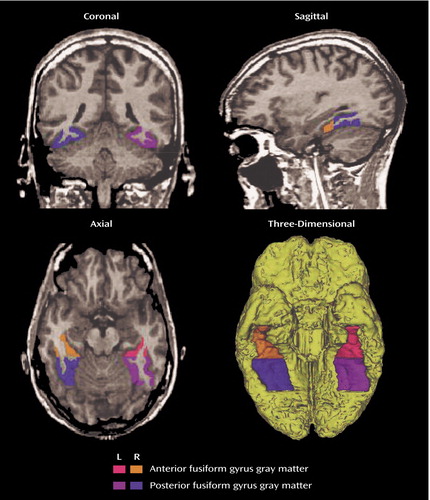
Figure 1. Delineation of the Anterior and Posterior Fusiform Gyrus Regions of Interest
1. Conklin HM, Calkins ME, Anderson CW, Dinzeo TJ, Iacono WG: Recognition memory for faces in schizophrenia patients and their first-degree relatives. Neuropsychologia 2002; 40:2314–2324Crossref, Medline, Google Scholar
2. Haxby JV, Hoffman EA, Gobbini MI: Human neural systems for face recognition and social communication. Biol Psychiatry 2002; 51:59–67Crossref, Medline, Google Scholar
3. McDonald B, Highley JR, Walker MA, Herron BM, Cooper SJ, Esiri MM, Crow TJ: Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: a postmortem study. Am J Psychiatry 2000; 157:40–47Link, Google Scholar
4. Onitsuka T, Shenton ME, Kasai K, Nestor PG, Toner SK, Kikinis R, Jolesz FA, McCarley RW: Fusiform gyrus volume reduction and facial recognition in chronic schizophrenia. Arch Gen Psychiatry 2003; 60:349–355Crossref, Medline, Google Scholar
5. Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, Dickey CC, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW: Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry 2002; 59:775–781Crossref, Medline, Google Scholar
6. Kwapil TR: Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. J Abnorm Psychol 1998; 107:558–565Crossref, Medline, Google Scholar
7. McCrae RR, Jang KL, Livesley WJ, Riemann R, Angleitner A: Sources of structure: genetic, environmental, and artifactual influences on the covariation of personality traits. J Pers 2001; 69:511–535Crossref, Medline, Google Scholar
8. Costa PT, McCrae RR: Revised NEO Personality Inventory (NEO-PI-R) and the NEO Five-Factor Inventory (NEO-FFI): Professional Manual. Odessa, Fla, Psychological Assessment Resources, 1992Google Scholar



