A Randomized, Controlled Trial of Cognitive Behavioral Social Skills Training for Middle-Aged and Older Outpatients With Chronic Schizophrenia
Abstract
OBJECTIVE: The number of older patients with chronic schizophrenia is increasing. There is a need for empirically validated psychotherapy interventions for these patients. Cognitive behavioral social skills training teaches cognitive and behavioral coping techniques, social functioning skills, problem solving, and compensatory aids for neurocognitive impairments. The authors compared treatment as usual with the combination of treatment as usual plus cognitive behavioral social skills training. METHOD: The randomized, controlled trial included 76 middle-aged and older outpatients with chronic schizophrenia, who were assigned to either treatment as usual or combined treatment. Cognitive behavioral social skills training was administered over 24 weekly group sessions. Blind raters assessed social functioning, psychotic and depressive symptoms, cognitive insight, and skill mastery. RESULTS: After treatment, the patients receiving combined treatment performed social functioning activities significantly more frequently than the patients in treatment as usual, although general skill at social functioning activities did not differ significantly. Patients receiving cognitive behavioral social skills training achieved significantly greater cognitive insight, indicating more objectivity in reappraising psychotic symptoms, and demonstrated greater skill mastery. The overall group effect was not significant for symptoms, but the greater increase in cognitive insight with combined treatment was significantly correlated with greater reduction in positive symptoms. CONCLUSIONS: With cognitive behavioral social skills training, middle-aged and older outpatients with chronic schizophrenia learned coping skills, evaluated anomalous experiences with more objectivity (achieved greater cognitive insight), and improved social functioning. Additional research is needed to determine whether cognitive insight mediates psychotic symptom change in cognitive behavior therapy for psychosis.
Effective psychotherapy interventions for middle-aged and older patients with chronic schizophrenia are needed. The number of middle-aged and older people with schizophrenia, like the number of older persons in the general population, is growing rapidly. By 2020, Americans over age 45 will constitute 41% of the entire population (1). This will mean a dramatic increase in demand for treatments targeting the unique needs of older patients with schizophrenia. Unfortunately, the likelihood of receiving any psychotherapy intervention declines dramatically in older patients (2), and the development and testing of psychosocial rehabilitation programs for older patients with schizophrenia have been slow.
One promising new approach to psychosocial treatment for patients with schizophrenia is to incorporate the techniques of cognitive behavior therapy. Several studies have shown that cognitive behavior therapy can improve symptom outcomes for patients with schizophrenia (for reviews, see references 3–7). While most studies of cognitive behavior therapy have focused on psychotic symptom outcomes, a few studies have shown improvement in social anxiety (8) and social functioning (9–11). Although psychotic symptoms remain important targets of pharmacologic and psychosocial interventions, the goals of treatments for serious mental illness have begun to focus more on improving functioning in social and instrumental role domains (12).
Social skills training is a related behavioral intervention for schizophrenia that focuses on social functioning (13, 14). Whereas cognitive behavior therapy focuses on how beliefs affect behavior and mood, social skills training focuses on practicing pragmatic skills of living. Benton and Schroeder (15) conducted a meta-analysis of 27 social skills training studies of younger schizophrenia patients and concluded that social skills training improved acquisition and durability of specific social skills. There was less evidence that social skills training improved relapse rates, symptom severity, or global social adjustment (16). It is possible that combining cognitive behavior therapy and social skills training may improve treatment of these other outcomes. For example, by challenging thoughts that interfere with skills execution in the real world (e.g., expectancies, delusional fears), social competence and functioning may be improved. Also, by adding social skills training, emphasis on social functioning is increased in types of cognitive behavior therapy that primarily target symptoms.
In prior studies of cognitive behavior therapy and social skills training, patients with schizophrenia were usually younger than 50 years old (3, 5, 7, 13, 15, 16). To address the lack of research on middle-aged and elderly patients, we developed a manualized intervention that combines cognitive behavior therapy and social skills training (17–19), with modifications addressing the unique needs of older psychiatric patients (20, 21) (e.g., interpersonal loss, physical disability, neurocognitive impairment, and patient belief systems such as, “I’m too old to learn”). Older patients with schizophrenia typically have not experienced institutionalization for many years, but about 60% live in assisted care settings (e.g., board-and-care homes) (22). Aging is typically associated with improvement in positive symptoms and reduced hospitalization rates (22), but most of these older people with schizophrenia have enduring neurocognitive impairments (23) that contribute to social functioning deficits (24–27). These patients report a strong desire to improve their social functioning (28). The primary target of the cognitive behavioral social skills training in the present study, therefore, was social functioning, and symptoms were secondary targets.
It is important to identify mechanisms of change in psychotherapy for psychosis. “Cognitive insight” is one factor recently described by Beck and colleagues (29) that may be related to symptom change in cognitive behavior therapy. In contrast to clinical insight, which typically refers to awareness of a mental illness requiring treatment, cognitive insight refers to metacognitive processes of reevaluation and correction of distorted beliefs and misinterpretations (e.g., objective distancing and reappraisal of symptoms). A patient may endorse a disease explanation for symptoms but may concurrently be unwilling to question long-held, emotionally charged delusional beliefs. A primary goal of cognitive behavior therapy for psychosis is to help patients to distance themselves from their distorted beliefs, more objectively evaluate evidence, accept corrective feedback about beliefs, and reduce overconfidence in conclusions. Patients who learn these skills may be more likely to show improvement in psychotic symptoms with cognitive behavior therapy. If improved cognitive insight is a mechanism of change in cognitive behavioral social skills training, patients receiving this intervention should show greater increases in cognitive insight than patients in treatment as usual, and patients receiving cognitive behavioral social skills training who show greater increases in cognitive insight should show greater reductions in psychotic symptoms.
This study was a randomized, controlled clinical trial of treatment as usual versus treatment as usual plus cognitive behavioral social skills training for middle-aged and older outpatients with very chronic schizophrenia. It included blind assessments of social functioning (primary outcomes), positive, negative, and depressive symptoms (secondary outcomes), cognitive insight, and mastery of skills addressed in cognitive behavioral social skills training (process variables). Assessments were obtained at baseline, midtreatment, and the end of treatment in order to examine the process of change through treatment. The timing of the assessments may also provide information about the amount of therapy that is needed to master skills and achieve meaningful change in outcomes. The primary study hypotheses were that patients receiving treatment as usual plus cognitive behavioral social skills training would show significantly better outcomes, especially in terms of social functioning, than patients receiving only treatment as usual and that change in cognitive insight would be related to change in symptom outcomes.
Method
Sample
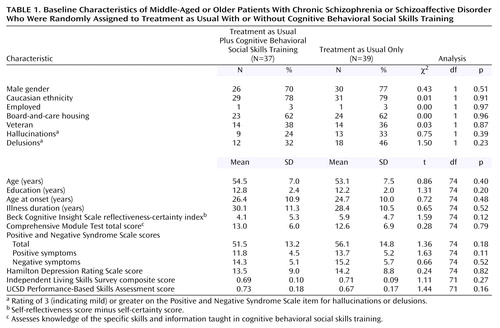
Community-dwelling patients with schizophrenia or schizoaffective disorder were recruited through the University of California, San Diego, Advanced Center for Interventions and Services Research for Psychosis in Older Adults. This was not a convenience sample. Participants were recruited from treatment and residential settings in San Diego County from 1999 to 2003. The participants were 42–74 years old, and the exclusion criteria were disabling medical problems that would interfere with testing, absence of medical records to inform diagnosis, and diagnosis of dependence on substances other than nicotine or caffeine within the past 6 months.
Diagnoses were based on the Structured Clinical Interview for DSM-IV (30). The diagnoses were as follows: schizophrenia, paranoid type, N=22; schizophrenia, undifferentiated type, N=22; schizophrenia, disorganized type, N=2; schizophrenia, residual type, N=2; and schizoaffective disorder, N=28. At baseline, 46 patients were taking one or more atypical antipsychotic medications, 17 were taking typical antipsychotics, seven were taking both typical and atypical medications, and six were not taking any antipsychotic medications. At baseline, 36 patients were also taking antidepressant medications, and 22 were taking mood stabilizers.
Study Design
This study was approved by the human subjects committees of the University of California, San Diego, and the Department of Veterans Affairs San Diego Healthcare System. Because transportation problems are common among older patients with schizophrenia, cognitive behavioral social skills training was delivered both at the research center (N=52) and at different board-and-care facilities in the community (N=24). Transportation to group therapy and to all assessment appointments at the research center was provided if requested. After the patients provided written informed consent and underwent baseline assessments, they were randomly assigned to one of two treatment conditions: 1) treatment as usual or 2) treatment as usual plus cognitive behavioral social skills training. They were followed up at midtreatment (3 months), to determine process and symptom outcomes, and at the end of treatment (6 months), to assess functioning, symptom, and process outcomes. A stratified randomization procedure was used to assign participants to treatments within sites, with the constraint that equal numbers of patients at each site would be assigned to the two conditions according to a sequential list of random numbers. The project coordinator assigned participants to treatments in the order that they consented, and the coordinator was the only staff person other than therapists with knowledge of group membership. The participants were compensated for the assessment visits but not for the treatment visits.
The assessors were blind to treatment group. Before each testing session, the patients met with the project coordinator, who counseled them not to reveal group membership to the raters. We assessed the blind by having assessors guess group membership at each assessment by using a 7-point Likert scale (–3 represented certainty that the intervention was treatment as usual, 0 represented complete uncertainty, and 3 represented certainty that the intervention was treatment as usual plus cognitive behavioral social skills training). The mean ratings were nearly 0 (completely unsure) for all three time points (0.03, 0.27, 0.60 for baseline, midtreatment, and end of treatment). For the vast majority of assessments, the raters were uncertain about group membership (the percentages of 1, 0, and –1 scores were 87%, 85%, and 73% for baseline, midtreatment, and end of treatment). Even in the minority of cases in which the assessors rated themselves as very certain about group membership (3 or –3 rating, 14% of assessments), they were wrong 36% of the time.
Outcome Measures
Primary outcomes
Social functioning was assessed by using the Independent Living Skills Survey (31) and the UCSD Performance-Based Skills Assessment (25). The Independent Living Skills Survey is a self-report measure of basic functional living skills performed during the past month; it was administered in an interview format. The UCSD Performance-Based Skills Assessment is a performance-based measure of the extent to which patients are capable of performing specific functional living skills, regardless of whether or not they actually performed the skill. This instrument assesses five domains of functioning—household chores, communication, finance, transportation, and planning recreational activities—by using standardized role-playing situations. The Independent Living Skills Survey assesses 10 domains, but the composite score computed was the average of scores on five relevant functional domains—appearance and clothing, personal hygiene, health maintenance, transportation, and leisure and community—that are sensitive to functional impairment in older outpatients with schizophrenia (26). Five domains were not used because the majority of participants in this study lived in board-and-care settings, where cleaning and cooking services (two domains) were provided, and almost all of the participants were retired or unemployed and receiving disability income that was managed by others (three domains). The participants, therefore, did not have the opportunity to perform these skills, so a score could not be computed accurately. The Independent Living Skills Survey and UCSD Performance-Based Skills Assessment were not administered to three participants, owing to an administrator error.
Secondary outcomes
The Positive and Negative Syndrome Scale (32) and Hamilton Depression Rating Scale (33) were also administered. Interrater reliability (interclass correlation) for the Positive and Negative Syndrome Scale total score was 0.88.
Process variables
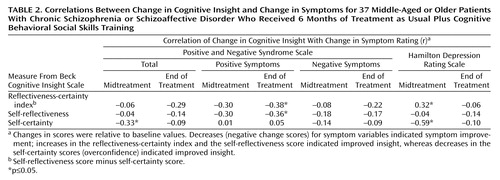
The Beck Cognitive Insight Scale (29) is a 15-item self-report inventory that includes two subscales, self-reflectiveness (objective reappraisal) and self-certainty (overconfidence in beliefs). A summary score is calculated by subtracting the self-certainty score from the self-reflectiveness score, and the difference is labeled the “reflectiveness-certainty index.”
The Comprehensive Module Test was used to assess knowledge of the specific skills and information taught in cognitive behavioral social skills training. The test was originally developed by the University of California, Los Angeles, Center for Research on Severe Mental Illness (14). The symptom self-management module test from the original Comprehensive Module Test was used to assess symptom knowledge and behavioral coping skills, as well as the ability to apply this knowledge in response to role playing and vignettes. Similar questions with vignettes were added to assess mastery of other skills addressed in cognitive behavioral social skills training, including communication, problem solving, and thought challenging. The total score on the Comprehensive Module Test was used (maximum=58).
Medications
Antipsychotic medication type (typical or atypical) and dose, other types of psychotropic medications (antidepressants, mood stabilizers), and any changes in medications were recorded. Psychiatrists and/or board-and-care workers verified medication information. The daily doses, in milligrams, of antipsychotic and anticholinergic medications at each assessment were calculated as chlorpromazine equivalents and benztropine equivalents, as clinically recommended (34–36).
Interventions
Treatment as usual
The patients continued in whatever ongoing care they were receiving. No medication guidelines were provided as part of this protocol. To characterize treatment as usual, a standardized service utilization interview was administered to all participants. Briefly, 82% of the patients reported a psychotropic medication visit in the 6 weeks preceding study entry, but only 19% of the patients reported receiving any type of psychotherapy (primarily group therapy) during the same period.
Cognitive behavioral social skills training
This intervention is described in detail elsewhere (17–19). The patients received 24 weekly 2-hour group psychotherapy sessions, with a half-hour lunch break (lunch was provided). The treatment manual included a patient workbook that contained homework forms. The components of social skills training were based on social skills training interventions available from Psychiatric Rehabilitation Consultants (37). The components of cognitive behavior therapy were developed specifically for patients with schizophrenia (38, 39). Aids to compensate for cognitive impairment, common in both schizophrenia and normal aging, were also added. The age-relevant content modifications included identifying and challenging ageist beliefs (e.g., “I’m too old to learn”), age-relevant role-playing situations (e.g., talking to a doctor about eyeglasses), and age-specific problem solving (e.g., finding transportation, coping with hearing and vision problems). Cognitive behavioral social skills training, therefore, targeted the multidimensional deficits that lead to disability in aging patients with schizophrenia.
An important obstacle to enrollment with traditional group therapy is treatment delays that occur when several patients must be recruited around the same time to start simultaneously. To avoid this, a modular design with rolling admissions was used. The participants started at the beginning of any of three four-session modules, so the maximum wait time was 4 weeks. The modules were self-contained, including orientation to the group in the first session and progression through skills across sessions. The patients completed all three modules twice, for a total of 24 sessions. The modules were repeated to compensate for cognitive impairment and to examine whether repeated exposure to the materials increased skill acquisition.
In the “thought-challenging module,” the patients used thought records and homework assignments to identify relationships among thoughts, feelings, and behaviors, and they identified mistakes in thinking. Patients conducted behavioral experiments to gather evidence to evaluate their beliefs. The primary thoughts targeted were beliefs about voices (e.g., “God is speaking,” “The voice could harm me”) and events related to delusions. To simplify learning and to help patients remember to use cognitive techniques in everyday life, mnemonic aids were provided (e.g., laminated wallet cards with “The 3Cs: Catch it—identify the thought, Check it—examine evidence, Change it.”).
The primary goal of the “asking for support module” was to improve communication skills and social interactions. This training used behavioral role-playing exercises that focused on reporting symptoms to doctors, expressing positive and negative feelings, assertive sharing in social interactions, and improving everyday leisure activities.
In the “solving problems module,” problem-solving skills were taught by using the acronym “SCALE,” which represented “Specify, Consider possible solutions, Assess the best solution, Lay out a plan, and Execute and evaluate the outcome.” Problems related to illness and disability were emphasized, including coping with symptoms, stressors (e.g., loss of a loved one), taking medication, using public transportation, leisure activities, hygiene and nutrition, and getting eyeglasses and hearing aids.
Therapist selection and quality assurance
Doctoral-level psychologists or senior graduate students in clinical psychology with at least master’s-level training and 2 years of clinical experience delivered the cognitive behavioral social skills training intervention (F.S.M., L.A.A., D.P., P.P., and others). Two therapists led each group. Two of us (E.G., J.R.M.) provided training and weekly supervision, including review of session videotapes. The Cognitive Therapy Rating Scale for Psychosis (40) was used to rate therapist competence from 30 videotaped sessions that were randomly selected but stratified according to module. The interrater reliability (interclass correlation) of the three raters was 0.85. The mean rating was 43 (SD=7). A score of 30 or more has been viewed as an adequate competence in cognitive behavior therapy for psychosis (41).
Statistical Analyses
Intent-to-treat analyses were used to examine all outcome variables. Missing data were replaced by within-group means of the missing values. All values for the Positive and Negative Syndrome Scale variables, Hamilton depression scale total, and Performance-Based Skills Assessment total had distributions that violated normality assumptions. Transformations (log, square root, or inverse) successfully normalized these scores. Differences between treatment groups at baseline were examined by using two-tailed t tests for continuous variables and chi-square tests for categorical variables. A split-plot repeated-measures analysis of covariance (ANCOVA) was used to test the difference between treatment groups at each follow-up point for each outcome measure, with the baseline score for the outcome measure as a covariate, treatment group (treatment as usual versus the combination of treatment as usual plus cognitive behavioral social skills training) and treatment delivery site (research center versus board-and-care facility) as between-subjects factors, and assessment time (midtreatment versus end of treatment) as a within-subjects factor. Predicted relationships between change in symptoms and change in cognitive insight were examined by computing Pearson’s r correlations between symptom change scores and insight change scores at midtreatment and at the end of treatment (relative to baseline).
Results
Sample Characteristics
Figure 1 shows patient flow through the study. Of the eligible patients, 72% consented to the study. Because eligible participants who refused to participate did not consent to any assessments, little is known about them, except that they had chart diagnoses of schizophrenia or schizoaffective disorder and were over age 40. Seventy-six patients were randomly allocated to treatments: 39 to treatment as usual and 37 to treatment as usual plus cognitive behavioral social skills training. At baseline, the two groups did not differ significantly on any demographic or outcome variable (Table 1). The majority of patients were high school educated, Caucasian, male, living in assisted housing, and nonveterans, and they had had schizophrenia or schizoaffective disorder for approximately three decades on average. At baseline, only 29% of the total sample reported at least mild hallucinations (i.e., had a Positive and Negative Syndrome Scale hallucinations item rating of 3, indicating mild, or higher), 40% reported at least mild delusions, and 40 (53%) reported at least mild delusions or hallucinations; the groups did not differ significantly on any of these positive symptom variables.
Treatment Adherence
Retention of participants at the end of treatment was 86% (Figure 1). Only four participants assigned to combined treatment failed to become engaged in treatment (two attended one session, one attended two, one attended four). Of the participants receiving combined treatment who engaged in treatment, 100% completed all three modules at least once (12 sessions), and 97% completed at least 18 (75%) of the sessions. For these participants who engaged in treatment, the mean number of sessions attended was 22 (92%), and the 95% confidence interval (CI) was 21–23; the mean percentage of homework assignments completed was 75% (95% CI=66%–84%, range=0%–100%). The group therapists also rated patient participation in group discussion on a 5-point Likert scale (1=none, 5=very active) at the end of each group session, and these ratings were averaged for each patient across all sessions. The mean participation rating was 3.8 (95% CI=3.6–4.0, range=2.9–5.0).
Outcomes
Marginal group means after treatment (averaged across sites and midtreatment/end-of-treatment assessment points), adjusted for baseline values, are presented in Figure 2 for each outcome measure. The treatment group effect was significant for frequency of social activities (Independent Living Skills Survey) (F=6.96, df=1, 68, p=0.02, η2=0.08), cognitive insight (Beck Cognitive Insight Scale) (F=9.91, df=1, 71, p=0.002, η2=0.12), and mastery of cognitive behavioral social skills training skills (Comprehensive Module Test) (F=34.78, df=1, 71, p<0.001, η2=0.33). When only engaged patients with complete data were included (on-protocol analysis), all of these treatment group effects were significant, and the effect sizes were comparable (Independent Living Skills Survey, η2=0.05; Beck Cognitive Insight Scale, η2=0.11; Comprehensive Module Test, η2=0.29). The treatment group effect was not significant for skill at performing basic everyday functioning activities (UCSD Performance-Based Skills Assessment) (F=3.92, df=1, 68, p=0.052, η2=0.05) or for any symptom measured with the Positive and Negative Syndrome Scale (positive symptoms: F=2.38, df=1, 71, p=0.13, η2=0.03; negative symptoms: F=0.43, df=1, 71, p=0.52, η2=0.01; total: F=1.28, df=1, 71, p=0.27, η2=0.02) or Hamilton depression scale (F=0.84, df=1, 71, p=0.37, η2=0.01). Psychiatric hospitalizations between baseline and the end of treatment were uncommon: two participants in treatment as usual were hospitalized (both for suicidal ideation), as were two receiving combined treatment (one for suicidal ideation and one for agitation/paranoia after clozapine was discontinued for medical reasons).
The group-by-time interaction was not significant for any outcome variable, indicating that the differences between treatment groups were similar at midtreatment and at the end of treatment for all outcome variables. The main effect of time (midtreatment versus end of treatment) also was not significant for any outcome variable. In addition, the group-by-site and group-by-site-by-time interactions were not significant for any outcome variable, indicating that the efficacy of the intervention was not significantly affected by site of delivery.
Cognitive Insight and Symptom Outcomes
For patients in treatment as usual plus cognitive behavioral social skills training, greater increases in the reflectiveness-certainty index and the self-reflectiveness subscale score were significantly correlated with a greater reduction in positive symptoms at the end of treatment (Table 2). In contrast, a greater increase in the reflectiveness-certainty index and a greater reduction in self-certainty (indicating better insight through reduced confidence in aberrant beliefs) were significantly correlated with increased depression at midtreatment but not by the end of treatment (Table 2). The only correlation that was significant for the group receiving treatment as usual was between an increased reflectiveness-certainty index and an increased score on the Hamilton depression scale at the 3-month (midtreatment) assessment (r=0.33, N=39, p<0.05). For the total sample at baseline, no significant correlation was found between any insight variable and any symptom variable (r=–0.16 to 0.17, N=76).
Medications
The treatment group effect at the end of treatment (determined by ANCOVA with the baseline value as a covariate) was not significant for the dose of antipsychotic medication or the dose of anticholinergic medication. For the group receiving combined treatment the mean chlorpromazine-equivalent dose of antipsychotic drugs was 425.0 mg/day (SD=381.5) at baseline and 455.8 (SD=300.2) at the end of treatment, whereas for the group receiving only treatment as usual the mean dose was 501.7 (SD=545.3) at baseline and 599.1 (SD=568.1) at the end of treatment (F=0.80, df=1, 73, p=0.38, η2=0.01). For the group receiving combined treatment the benztropine-equivalent dose of anticholinergic drugs was 1.2 mg/day (SD=1.7) at baseline and 1.3 (SD=2.2) at the end of treatment, and for the group receiving only treatment as usual the mean dose was 1.8 (SD=2.3) at baseline and 2.3 (SD=2.4) at the end of treatment (F=2.14, df=1, 73, p=0.15, η2=0.03). The groups also did not differ significantly in the numbers of patients starting antipsychotic treatment or increasing the dose (combined treatment, N=15; treatment as usual, N=18), adding an atypical antipsychotic (combined treatment, N=2; treatment as usual, N=3), starting mood medication (combined treatment, N=2; treatment as usual, N=5), or discontinuing mood medication (combined treatment, N=7; treatment as usual, N=7).
Discussion
This randomized clinical trial examined whether adding a cognitive behavioral group psychotherapy intervention to treatment as usual improved functional outcome in middle-aged and older patients with schizophrenia or schizoaffective disorder. Except for pilot studies at our research center, we are aware of no other published clinical trial of a psychotherapy intervention for older psychotic patients. After treatment, the patients who received treatment as usual plus cognitive behavioral social skills training performed social functioning activities significantly more frequently than patients in treatment as usual. The significant improvement found in everyday functioning is important in this patient population, given that despite relatively effective pharmacologic control of psychiatric symptoms, impairments in social functioning persist in these patients.
The treatment groups did not differ significantly in their general skill at performing specific everyday functioning activities after treatment (measured with the UCSD Performance-Based Skills Assessment). Cognitive behavioral social skills training does not specifically train all the skills measured by this instrument, and this may have contributed to the lack of improvement on this measure. Although proficiency at performing functional activities did not improve significantly in the group receiving combined treatment, the Independent Living Skills Survey showed that these participants were significantly more likely than participants in treatment as usual to actually perform social functioning activities after therapy. A focus of cognitive behavioral social skills training is challenging the thoughts that interfere with execution of activities in the community (e.g., “I will be harmed if I go out,” “It won’t be fun,” “I won’t be able to do it”). By challenging illness-related thoughts (e.g., paranoia) and thoughts that interfere with the execution of everyday activities, patients were more likely to engage in social activities.
Despite older age, chronic severe mental illness for approximately three decades, and neurocognitive deficits common in older patients with schizophrenia, the patients receiving cognitive behavioral social skills training had excellent group attendance, homework completion, and participation and they were able to learn the content of the intervention. Providing transportation and conducting the intervention at board-and-care facilities in the community likely contributed to the excellent attendance and low dropout rate. It is important that the site of treatment delivery did not significantly affect any treatment outcome variable. Cognitive behavioral social skills training was equally effective delivered in the community and at a major medical center. Interventions developed for older psychiatric patients must be tailored to the unique needs of this population and reduce barriers to treatment in this way.
The results of this study are consistent with those of several other studies in which significant improvement in psychosocial functioning occurred after cognitive behavior therapy for younger patients with schizophrenia (9–11). These promising results suggest that aspects of cognitive behavior therapy that specifically target psychosocial functioning (e.g., social skills training, problem-solving training, and challenges to thoughts that interfere with skill execution) should be strengthened and that social functioning outcome measures should be included in clinical trials. Given that antipsychotic medications reduce psychotic symptom severity but do not specifically target everyday functioning, cognitive behavior therapy interventions that focus on functioning may be particularly important for schizophrenia patients, especially older, very chronically ill community-dwelling patients.
The present study did not show a significant treatment group effect on positive or negative symptoms or depression. In general, studies of cognitive behavior therapy for schizophrenia have shown significant improvement in positive and/or negative symptoms in younger, treatment-resistant subjects (3–7). The absence of significant benefit for symptoms in this study was probably due to the fact that symptoms had been well controlled by medications at baseline. Therapist inexperience also may have influenced the results for positive symptoms in this study. Master’s- and doctoral-level therapists were used in this study, whereas U.K. studies that showed greater impact on symptoms most commonly used more experienced doctoral-level therapists (5), although one study with positive findings used psychiatric nurses (42). Research is needed to examine the replicability and generalizability of cognitive behavior therapy for schizophrenia with clinicians in community settings who are not the experts who developed the treatment. The present study was an important step in that direction, because some group sessions were conducted at community sites by less experienced therapists.
The majority of prior studies of cognitive behavior therapy for schizophrenia used an individual therapy format, but some promising results have been reported from using a group format (8, 11, 43) or combined group and individual therapy (44). One important difference between group and individual cognitive behavior therapy is that groups tend to emphasize skills training over detailed case formulation. There is less time in groups to fully explore the unique content and history of each patient’s delusional system and hallucinations. This may have contributed to the lack of significant impact on symptoms in this study. Groups, however, can influence social support systems and allow the practice of communication and other social skills with peers, which may be important for interventions, such as cognitive behavioral social skills training, that target social functioning. Future studies might compare interventions that use a comprehensive case formulation with interventions that do not, in order to determine the importance of this approach for different patient subgroups and different outcomes.
Improvement in cognitive insight (29) may be one mechanism of symptom change in cognitive behavior therapy. The patients who received treatment as usual plus cognitive behavioral social skills training showed significantly greater cognitive insight after treatment than did the patients in treatment as usual. There was also some evidence that the treatment group effect on psychiatric symptoms was related to changes in cognitive insight. Improvement in overall cognitive insight (Beck Cognitive Insight Scale reflectiveness-certainty index) was significantly correlated with reduction in positive symptoms at the end of treatment only for the patients who received cognitive behavioral social skills training. This association appeared to be driven by increased self-reflectiveness (i.e., increased objective examination of evidence for beliefs). In contrast, improvement in overall cognitive insight was associated at midtreatment with a transient increase in depression, which resolved by the end of treatment. This association appeared to be driven by decreased self-certainty (i.e., reduced overconfidence in conclusions about anomalous experiences). Several of these correlations, however, were exploratory. If they are replicated, future research on cognitive insight as a possible mediator of symptom outcome in cognitive behavior therapy, particularly positive symptom outcome, may help clarify the higher-level cognitive (or metacognitive) processes by which cognitive behavior therapy can lead to changes in symptoms. Among the patients in cognitive behavioral social skills training, those who became more objective, more willing to examine cognitive distortions, and less resistant to corrective feedback from others (i.e., increased self-reflectiveness) showed greater reduction in positive symptoms. Patients can, however, become depressed as their confidence in distorted long-held beliefs is reduced (i.e., reduced self-certainty) and they begin to understand their anomalous experiences as part of chronic illness. Clinicians using cognitive therapy techniques should be aware of this and plan for the possibility of addressing increased depression in therapy.
The analyses did not reveal any significant benefit from repeating the intervention modules a second time (i.e., no significant additional improvement from midtreatment to the end of treatment). The duration of treatment in previous clinical trials of cognitive behavior therapy for psychosis ranged from 5 weeks to 9 months and averaged 13.6 weeks (5). Shorter treatments have been used in acute illness phases, whereas longer durations, such as for group cognitive behavioral social skills training, have been used for more chronically ill populations. The present findings may indicate that 3 months (one exposure to the modules) is a sufficient dose of group cognitive behavioral social skills training for chronically ill older patients. It is possible, however, that repetition was important for maintaining the gains achieved by midtreatment. This finding also may not generalize to other forms of cognitive behavior therapy or other samples, and it is not clear whether a subgroup of patients with more severe cognitive deficits would require a longer course of cognitive behavioral social skills training to acquire the skills and show benefit.
The strengths of the study included randomization to treatments, blind raters, good matching of groups at baseline on all variables, a manualized intervention, monitoring of treatment fidelity, broad outcome measures, good attendance and retention of participants, and inclusion of midtreatment assessments and cognitive insight variables to examine possible mechanisms of change. The limitations included a moderately small sample size and lack of a control for nonspecific therapist contact factors, although other studies of cognitive behavior therapy for schizophrenia have demonstrated its efficacy relative to control conditions consisting of supportive contact (41, 42). Exclusion of patients with current comorbid substance dependence may also reduce the generalization of the findings, although comorbid substance dependence declines with age in patients with schizophrenia (45). In addition, only ratings of symptom severity (e.g., Positive and Negative Syndrome Scale) were obtained, and ratings of distress or dysfunction due to symptoms (e.g., Psychotic Symptom Rating Scales [46]) were not collected. It is possible that these symptom dimensions change independently with cognitive behavior therapy. For example, Bach and Hayes (47) noted that patients with schizophrenia receiving cognitive behavior therapy actually had increases in their reported frequency and severity of symptoms but that they reported less distress and dysfunction related to their symptoms. This finding is not surprising given that cognitive behavior therapy typically teaches patients to monitor symptoms, increase awareness of symptoms, and even practice talking about symptoms with professionals in role playing. By modifying beliefs about symptoms, the distress and dysfunction associated with symptoms can be reduced, despite the persistence of the symptoms themselves.
In conclusion, to our knowledge this is the first published randomized trial to examine a psychosocial intervention designed for the unique needs of older patients with psychotic disorders. This study adds to the growing evidence of the efficacy of cognitive therapy interventions in schizophrenia. Given the heterogeneity of schizophrenia, it is unlikely that a single cognitive behavior therapy intervention will work equally well for all types of patients (e.g., young and old, acutely and chronically ill, medication-resistant and -responsive, neurocognitively normal and impaired, insightful and unaware). Researchers should continue to develop and test group and individual cognitive behavior therapy interventions that are tailored to the unique needs of different subgroups of patients with schizophrenia and identify which treatments are most effective for which patients and in what circumstances.
 |
 |
Presented in part at the 17th annual meeting of the Society for Research in Psychopathology, San Francisco, Sept. 26–29, 2002, and the 37th annual meeting of the Association for Advancement of Behavior Therapy, Boston, Nov. 20–23, 2003. Received Dec. 4, 2003; revision received March 15, 2004; accepted April 5, 2004. From the Psychology Service, Department of Veterans Affairs San Diego Health Care System; the Department of Psychiatry and Department of Neurosciences, University of California, San Diego; and the San Diego State University/UCSD Joint Doctoral Program in Clinical Psychology. Address correspondence and reprint requests to Dr. Granholm, VA San Diego Healthcare System (116B), 3350 La Jolla Village Dr., San Diego, CA 92161; [email protected] (e-mail). Supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, by NIMH grants R01 MH-61381 and 1P30 MH-066248, and by the National Alliance for Research on Schizophrenia and Depression.
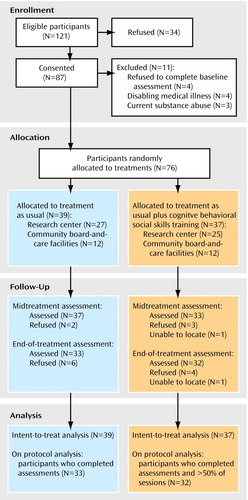
Figure 1. Flow of Middle-Aged or Older Patients With Chronic Schizophrenia or Schizoaffective Disorder Through a 6-Month Study of Treatment as Usual With or Without Cognitive Behavioral Social Skills Training
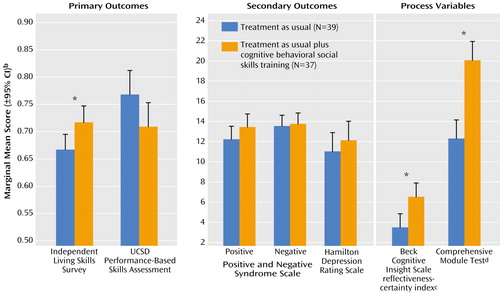
Figure 2. Outcome Measures for Middle-Aged or Older Patients With Chronic Schizophrenia or Schizoaffective Disorder Who Received 6 Months of Treatment as Usual With or Without Cognitive Behavioral Social Skills Traininga
aThe scores were averaged across sites and across assessment times (midtreatment and end of treatment) and adjusted for baseline. Effects of treatment group were determined by ANOVAs.
bAdjusted for baseline value.
cSelf-reflectiveness score minus self-certainty score.
dAssesses knowledge of the specific skills and information taught in cognitive behavioral social skills training.
*p≤0.05.
1. Jeste DV, Alexopoulos GS, Bartels SJ, Cummings JL, Gallo JJ, Gottlieb GL, Halpain MC, Palmer BW, Patterson TL, Reynolds CF III, Lebowitz BD: Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry 1999; 56:848–853Crossref, Medline, Google Scholar
2. Lehman AF, Steinwachs DM: Patterns of usual care for schizophrenia: initial results from the Schizophrenia Patient Outcomes Research Team (PORT) client survey. Schizophr Bull 1998; 24:11–20Crossref, Medline, Google Scholar
3. Cormac I, Jones C, Campbell C: Cognitive behaviour therapy for schizophrenia. Cochrane Database Syst Rev 2002, issue 1, CD000524Google Scholar
4. Dickerson FB: Cognitive behavioral psychotherapy for schizophrenia: a review of recent empirical studies. Schizophr Res 2000; 43:71–90Crossref, Medline, Google Scholar
5. Gould RA, Mueser KT, Bolton E, Mays V, Goff D: Cognitive therapy for psychosis in schizophrenia: an effect size analysis. Schizophr Res 2001; 48:335–342Crossref, Medline, Google Scholar
6. Haddock G, Tarrier N, Spaulding W, Yusupoff L, Kinney C, McCarthy E: Individual cognitive behavior therapy in the treatment of hallucinations and delusions: a review. Clin Psychol Rev 1998; 18:821–838Crossref, Medline, Google Scholar
7. Rector NA, Beck AT: Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis 2001; 189:278–287Crossref, Medline, Google Scholar
8. Kingsep P, Nathan P, Castle D: Cognitive behavioural group treatment for social anxiety in schizophrenia. Schizophr Res 2003; 63:121–129Crossref, Medline, Google Scholar
9. Wiersma D, Jenner JA, van de WG, Spakman M, Nienhuis FJ: Cognitive behaviour therapy with coping training for persistent auditory hallucinations in schizophrenia: a naturalistic follow-up study of the durability of effects. Acta Psychiatr Scand 2001; 103:393–399Crossref, Medline, Google Scholar
10. Bradshaw W: Integrating cognitive behavioral psychotherapy for persons with schizophrenia into a psychiatric rehabilitation program: results of a three year trial. Community Ment Health J 2000; 36:491–500Crossref, Medline, Google Scholar
11. Gumley A, O’Grady M, McNay L, Reilly J, Power K, Norrie J: Early intervention for relapse in schizophrenia: results of a 12-month randomized controlled trial of cognitive behavioural therapy. Psychol Med 2003; 33:419–431Crossref, Medline, Google Scholar
12. Breier A, Schreiber J, Dyer J, Pickar D: National Institute of Mental Health longitudinal study of chronic schizophrenia: prognosis and predictors of outcome. Arch Gen Psychiatry 1991; 48:239–246Crossref, Medline, Google Scholar
13. Heinssen RK, Liberman RP, Kopelowicz A: Psychosocial skills training for schizophrenia: lessons from the laboratory. Schizophr Bull 2000; 26:21–46Crossref, Medline, Google Scholar
14. Liberman RP: Psychosocial treatments for schizophrenia. Psychiatry 1994; 57:104–114Crossref, Medline, Google Scholar
15. Benton MK, Schroeder HE: Social skills training with schizophrenics: a meta-analytic evaluation. J Consult Clin Psychol 1990; 58:741–747Crossref, Medline, Google Scholar
16. Pilling S, Bebbington P, Kuipers E, Garety P, Geddes J, Orbach G, Morgan C: Psychological treatments in schizophrenia, I: meta-analysis of family intervention and cognitive behaviour therapy. Psychol Med 2002; 32:763–782Medline, Google Scholar
17. Granholm E, McQuaid JR, Auslander L, McClure FS: Group cognitive behavioral social skills training for older outpatients with chronic schizophrenia. J Cognitive Psychotherapy: An International Quarterly 2004; 18:265–279Crossref, Google Scholar
18. Granholm E, McQuaid JR, McClure FS, Pedrelli P, Jeste DV: A randomized controlled pilot study of cognitive behavioral social skills training for older patients with schizophrenia. Schizophr Res 2002; 53:167–169Crossref, Medline, Google Scholar
19. McQuaid JR, Granholm E, McClure FS, Roepke S, Pedrelli P, Patterson TL, Jeste DV: Development of an integrated cognitive behavioral and social skills training intervention for older patients with schizophrenia. J Psychother Pract Res 2000; 9:149–156Medline, Google Scholar
20. Arean PA, Cook BL, Gallagher-Thompson D, Hegel MT, Schulberg HC, Schulz R: Guidelines for conducting geropsychotherapy research. Am J Geriatr Psychiatry 2003; 11:9–16Crossref, Medline, Google Scholar
21. Gallagher-Thompson D, Thompson KW: Psychotherapy with older adults in theory and practice, in Comprehensive Textbook of Psychotherapy: Theory and Practice. Edited by Bongar BM, Beutler LE, Bongar B. New York, Oxford University Press, 1995, pp 359–379Google Scholar
22. Jeste DV, Twamley EW, Eyler Zorrilla LT, Golshan S, Patterson TL, Palmer BW: Aging and outcome in schizophrenia. Acta Psychiatr Scand 2003; 107:336–343Crossref, Medline, Google Scholar
23. Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV: Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry 2001; 58:24–32Crossref, Medline, Google Scholar
24. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Link, Google Scholar
25. Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste D: UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull 2001; 27:235–245Crossref, Medline, Google Scholar
26. Perivoliotis D, Granholm E, Patterson TL: Psychosocial functioning on the Independent Living Skills Survey in older outpatients with schizophrenia. Schizophr Res 2004; 69:307–316Crossref, Medline, Google Scholar
27. Twamley EW, Doshi RR, Nayak GV, Palmer BW, Golshan S, Heaton RK, Patterson TL, Jeste DV: Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry 2002; 159:2013–2020Link, Google Scholar
28. Auslander LA, Jeste DV: Perceptions of problems and needs for service among middle-aged and elderly outpatients with schizophrenia and related psychotic disorders. Community Ment Health J 2002; 38:391–402Crossref, Medline, Google Scholar
29. Beck AT, Baruch E, Balter JM, Steer RA, Warman DM: A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res 2004; 68:319–329Crossref, Medline, Google Scholar
30. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1994Google Scholar
31. Wallace CJ, Liberman RP, Tauber R, Wallace J: The Independent Living Skills Survey: a comprehensive measure of the community functioning of severely and persistently mentally ill individuals. Schizophr Bull 2000; 26:631–658Crossref, Medline, Google Scholar
32. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
33. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
34. Jeste DV, Wyatt R: Understanding and Treating Tardive Dyskinesia. New York, Guilford, 1982, p 3Google Scholar
35. Post RM: Mood disorders: treatment of bipolar disorders, in Kaplan and Sadock’s Comprehensive Textbook of Psychiatry, 7th ed. Edited by Sadock BJ, Sadock VA. Philadelphia, Lippincott Williams & Wilkins, 2000, pp 1385–1430Google Scholar
36. Mcyer JM, Simpson GM: Anticholinergics and amantadine. Ibid, pp 2276–2282Google Scholar
37. Modules in the UCLA Social and Independent Living Skill Series. Camarillo, Calif, Psychiatric Rehabilitation Consultants, 1991Google Scholar
38. Kingdon DG, Turkington D: Cognitive Behavioral Therapy of Schizophrenia. New York, Guilford, 2002Google Scholar
39. Beck AT, Rector NA: Cognitive therapy of schizophrenia: a new therapy for the new millennium. Am J Psychother 2000; 54:291–300Crossref, Medline, Google Scholar
40. Haddock G, Devane S, Bradshaw T, McGovern J, Tarrier N, Kinderman P, Baguley I, Lancashire S, Harris N: An investigation into the psychometric properties of the Cognitive Therapy Scale for Psychosis (CTS-Psy). Behavioural & Cognitive Psychotherapy 2001; 29:221–233Crossref, Google Scholar
41. Turkington D, Kingdon D, Turner T: Effectiveness of a brief cognitive behavioural therapy intervention in the treatment of schizophrenia. Br J Psychiatry 2002; 180:523–527Crossref, Medline, Google Scholar
42. Sensky T, Turkington D, Kingdon D, Scott JL, Scott J, Siddle R, O’Carroll M, Barnes TR: A randomized controlled trial of cognitive behavioral therapy for persistent symptoms in schizophrenia resistant to medication. Arch Gen Psychiatry 2000; 57:165–172Crossref, Medline, Google Scholar
43. Wykes T, Parr AM, Landau S: Group treatment of auditory hallucinations: exploratory study of effectiveness. Br J Psychiatry 1999; 175:180–185Crossref, Medline, Google Scholar
44. Drury V, Birchwood M, Cochrane R, Macmillan F: Cognitive therapy and recovery from acute psychosis: a controlled trial, I: impact on psychotic symptoms. Br J Psychiatry 1996; 169:593–601Crossref, Medline, Google Scholar
45. Palmer BW, Heaton SC, Jeste DV: Older patients with schizophrenia: challenges in the coming decades. Psychiatr Serv 1999; 50:1178–1183Link, Google Scholar
46. Haddock G, McCarron J, Tarrier N, Faragher EB: Scales to measure dimensions of hallucinations and delusions: the Psychotic Symptom Rating Scales (PSYRATS). Psychol Med 1999; 29:879–889Crossref, Medline, Google Scholar
47. Bach P, Hayes SC: The use of acceptance and commitment therapy to prevent the rehospitalization of psychotic patients: a randomized controlled trial. J Consult Clin Psychol 2002; 70:1129–1139Crossref, Medline, Google Scholar



