Auditory Startle Response in Firefighters Before and After Trauma Exposure
Abstract
OBJECTIVE: Although previous psychophysiological studies have revealed heightened muscular and autonomic responses in individuals with posttraumatic stress disorder (PTSD), these studies have not permitted inferences about whether the abnormal responses are a vulnerability factor or are acquired following trauma. The present study reports the first prospective psychophysiological investigation, to the authors’ knowledge, of posttraumatic stress responses by prospectively evaluating the auditory startle response in firefighters before and after trauma exposure. METHOD: Orbicularis oculi (eye blink) electromyograms and skin conductance responses to 15 100-dB acoustic startle stimuli were assessed in 84 trainee firefighters before trauma exposure. After commencement of active duty, 35 firefighters were reassessed within 4 weeks of exposure to a traumatic event, and 36 firefighters were reassessed as a comparison group that was not exposed to trauma. RESULTS: In the trauma-exposed group, pretrauma physiological activity was predictive of posttrauma acoustic startle responses. Pretrauma skin conductance response to startle was also predictive of posttraumatic stress severity. CONCLUSIONS: These results provide initial support for elevated startle response being a vulnerability factor for posttraumatic stress responses.
There is strong evidence that posttraumatic stress disorder (PTSD) is characterized by elevated arousal (1, 2). One indicator of hyperarousal is exaggerated startle response. Reflecting the acoustic startle reflex, larger magnitude orbicularis oculi (eye blink) electromyograms (EMGs), skin conductance responses, and elevated heart rate responses, more slowly habituating skin conductance responses to startle stimuli, have often been reported in individuals with PTSD (3–8).
Biological models posit that sympathetic arousal elicited during the trauma triggers the release of stress hormones (including norepinephrine, epinephrine, and cortisol) that may result in exaggerated reactivity to stimuli and may contribute to subsequent PTSD (9). Two studies have found that resting heart rates in the week after trauma were greater in those who subsequently developed PTSD than those who did not (10, 11), although a recent study failed to replicate these findings (12). In addition, the development of PTSD is associated with reduced cortisol levels in the acute phase after trauma (13). It has been proposed that cortisol functions to contain a series of biological reactions in the hypothalamic-pituitary-adrenal axis, and therefore lower cortisol levels contribute to elevated arousal (14). Finally, elevated heart rate and more slowly habituating skin conductance and EMG responses to startle stimuli have been observed 1 month after trauma but not 1 week after trauma in those who developed PTSD (8). This finding accords with a theory of progressive neuronal sensitization associated with heightened physiological reactivity underlying PTSD development (8, 15).
An outstanding issue in the study of posttraumatic stress is whether heightened physiological responding associated with PTSD is constitutional or acquired. In an attempt to address this issue, a recent study (16) compared startle responses in pairs of Vietnam combat veterans and their non-combat-exposed monozygotic twins. Elevated heart rate responses to startle were evident in veterans with PTSD but not in their non-combat-exposed co-twins. This finding was hypothesized to reflect an acquired sign of PTSD, however, the notion that elevated heart rate responses to startle stimuli represent an inherited predisposition requiring trauma exposure to be switched on could not be addressed within the study’s design (16). There was some indication that more slowly habituating skin conductance responses to startle stimuli may represent a pretrauma vulnerability factor for PTSD (16).
The proposition that heightened physiological response to startle stimuli reflects a pretrauma vulnerability factor for posttraumatic stress (17) has never been tested prospectively. Although there is indirect evidence from the studies by Shalev et al. (8) and Orr et al. (16) that startle reactivity may be acquired posttrauma, there is a lack of prospective research supporting this notion. Accordingly, the aim of the present study was to examine the relationship between the auditory startle response and psychopathology before and after trauma exposure. If heightened startle reactivity reflects a vulnerability factor, elevated startle response before trauma exposure should predict posttraumatic stress. The relationship between pre- and posttrauma psychophysiology and psychopathology was evaluated in the current study within the context of some other known predictors of PTSD (e.g., previous trauma, prior PTSD, preexisting anxiety or depression, and trauma severity; see reference 18). Pretrauma pattern of alcohol consumption was also considered, given recent evidence of a shared genetic vulnerability between alcohol use and PTSD (19, 20).
Method
Subjects
Eligible subjects were 87 successive male recruits (mean age=30.1 years, SD=5.1) to the New South Wales Fire Brigades who were receiving class-based instruction at the time of recruitment; three subjects (3%) declined to participate in the study. The subjects were initially evaluated before commencing active firefighting duties (time 1), and then attempts were made to reassess all subjects within 12 months of the initial assessment (time 2). The experimenters monitored the subjects’ trauma exposure on a weekly basis with the New South Wales Fire Brigade’s emergency calls database. In total, 71 subjects (85%) were reevaluated within 12 months of the commencement of active firefighting duty. Nonparticipation occurred because one participant had moved to a rural area, two were receiving workers’ compensation related to a physical injury, and 10 refused to be reassessed. The subjects who participated in the follow-up assessment did not differ from the nonparticipants on age or initial psychometric responses.
At follow-up, 35 subjects were reassessed between 2 and 28 days (mean=14.0, SD=8.2) after experiencing a trauma and were classified into the trauma-exposed group. Thirty-six subjects at follow-up had not been exposed to a trauma and were classified into the non-trauma-exposed group. An event was defined as a stressor if it satisfied the DSM-IV description of criterion A of the PTSD criteria. The traumatic events related to the fire brigade experienced by the subjects in the trauma-exposed group included motor vehicle accidents (N=27, 77%), fires (N=6, 17%), and attending the scene of a suicide (N=2, 26%). One subject in each group was taking antihypertensive medication at the time of both assessments.
Psychometrics
At the initial assessment, a master’s level psychologist administered the subjects the Structured Clinical Interview for DSM-IV (SCID) (21) to look for current axis I disorders and the Clinician Administered PTSD Scale (22, 23) to index current posttraumatic stress symptoms. The subjects were also administered the Traumatic Events Questionnaire (24) to assess prior traumatic events, the trait version of the State-Trait Anxiety Inventory (25) to assess trait anxiety, the Beck Anxiety Inventory (26) to index state anxiety, the Beck Depression Inventory II (27) to index depressive symptoms, the Dissociative Experiences Scale—Taxon (28), a subscale of the Dissociative Experiences Scale (29) that measures pathological dissociation, and the Alcohol Use Disorders Identification Test (30) to assess current alcohol use.
At time 2, the subjects were readministered the SCID, the Beck Depression Inventory II, the Beck Anxiety Inventory, the trait version of the State-Trait Anxiety Inventory, and the Alcohol Use Disorders Identification Test. In addition, they were administered the Acute Stress Disorder Interview (31) to assess acute stress disorder, the Impact of Event Scale (32) to assess intrusive and avoidance symptoms, the Peritraumatic Dissociative Experiences Questionnaire (33) to index dissociative responses during and immediately after the trauma, and the Social Desirability Scale (34) to assess the subjects’ need to respond to self-report items in culturally acceptable ways. Trauma-exposed subjects were also asked to rate the severity of the event they were exposed to (0=not at all traumatic, 100=very traumatic). (The non-trauma-exposed subjects were administered the Acute Stress Disorder Interview, the Impact of Event Scale, and the Peritraumatic Dissociative Experiences Questionnaire in relation to the most stressful event they had experienced during the preceding 4-week period.)
Stimuli
Experimental stimuli consisted of 15 consecutive 100-dB (sound-pressure level), 500-msec, 1000-Hz pure tones, with instantaneous rise and fall times. The tones were generated by a Coulbourn audio source module (V85-05, Coulbourn Instruments, Allentown, Pa.) and a stereo amplifier and were presented binaurally through stereo headphones. A sound level and an artificial ear were used for calibration of stimulus intensity. Intertrial intervals were randomly selected by a computer and ranged from 30 to 55 seconds.
Physiological Measures
Physiological data, consisting of left orbicularis oculi EMG and skin conductance measures, were recorded employing a Coulbourn lablinc V system. EMGs were recorded through 4-mm (sensor diameter) silver/silver chloride surface electrodes filled with Microlyte electrolytic gel (Coulbourn Instruments) and attached with adhesive collars over the left orbicularis oculi, in accordance with recommendations by Fridlund and Cacioppo (35). The raw EMG signal was amplified by 50,000 and filtered to retain the 90–1000-Hz frequency range by a Coulbourn isolated bioamplifier (V75-04). The EMG signal was rectified and integrated by a Coulbourn multifunction integrator (V76-23A) by using a 10-msec time constant. Skin conductance was measured directly by a Coulbourn isolated skin conductance coupler (V71-23) by using a constant 0.5 V through 8-mm silver/silver chloride electrodes. Electrodes were filled with Microlyte gel and attached with adhesive collars to the subjects’ nondominant distal phalanges of the index and middle fingers, in accordance with published guidelines (36). The saline concentration of Microlyte gel (1.34 mol) is not near saturation point, in compliance with published recommendations (37, 38); however, it is higher than the recommended concentration for skin conductance recording (0.05 mol [36]). The Coulbourn instruments were interfaced with a computer through a Coulbourn general-purpose lablinc port (V19-16).
Procedure
All procedures were conducted in a 3×2.5-m temperature-controlled room and connected by wires to an adjoining room in which the experimental apparatus was located. The subjects were seated in a comfortable armchair and monitored by an unobtrusive video camera. After completion of the written informed consent procedures and the initial assessment, the psychophysiological recording equipment was attached. The subjects were asked to sit quietly for a 5-minute resting period, during which time the physiological measures were sampled continuously at the rate of 2 Hz. After the baseline period, the subjects’ basal tonic level was balanced with a voltmeter. The subjects were asked to rate their current mood (0=very happy, 12=very sad) and anxiety level (0=very calm, 12=very anxious) and were then given the following instructions, based on those used by Shalev et al. (4): “Shortly you are going to hear a series of sounds. Please sit quietly and listen to the sounds as they come. Keep your eyes open throughout the entire procedure, which will last approximately 15 minutes.” Physiological analogue signals were digitized at 1 kHz throughout the startle procedure.
Response Scores
Peak magnitude of the orbicularis oculi EMG response to the acoustic startle probe for each trial was calculated by subtracting the average EMG level for the 20 msec after tone onset from the maximum level within 21 to 200 msec of tone onset. Trials during which a voluntary or spontaneous blink occurred within 20 msec of the stimulus onset were omitted from further analysis because of evidence that blink reflexes typically do not occur within 20 msec (39, 40). In total, 3% of eye blinks were rejected because of excessive noise during the baseline period (e.g., spontaneous blinks or unusually high amounts of integrated EMG during baseline) or because the onset of EMG eye blink response began less than 20 msec after tone onset. Rejected trials were treated as missing data and were assigned a mean response score based on the trial preceding and following the missing value. There were no more than three missing EMG values within either assessment for any participant. Trials with no perceptible eye blink reflex (10% of all trials) were assigned a magnitude of zero and were included in the analysis, following a protocol outlined by previous investigators (40, 41). A skin conductance response score was calculated for each trial by subtracting the average skin conductance level for the 1 second immediately preceding tone onset from the maximum level within 1 to 4 seconds after tone onset (4, 5). To reduce the variance associated with unusually large responses, square root transformations were performed on the EMG and skin conductance response scores before analysis (4, 5).
Habituation Measures
Habituation was assessed by using two methods (4, 5). First, a measure of relative habituation was computed from the slope of the regression equation Y=bX+a for trials 2–15, where Y is the square root of the response score and X is the log of the trial number. The first trial was excluded from computation of the habituation curve because its skin conductance response can be smaller than the skin conductance response to the second trial (5). Second, the number of trials taken to reach a criterion of two successive nonresponse trials was computed for each participant for EMG and skin conductance responses as a measure of absolute habituation. A nonresponse trial was defined for EMG as a response score ≤3.0 μV and for skin conductance as a response score ≤0.05 μS (cutoff values were based on untransformed data) (5). (There were no perceptible eye blinks below 3.0 μV with the hardware settings employed in this study.) Trials to nonresponse scores ranged from 0 (nonresponses to the first two trials) to 14 (criterion of two successive nonresponse trials not met).
Results
Demographic Characteristics and Psychometrics
Group mean demographic and psychometric data at times 1 and 2, with analyses of variance and analysis of covariance (ANCOVA), are presented in Table 1. Planned multiple comparisons (that adopted an adjusted alpha of 0.01) at time 1 indicated that the trauma-exposed and non-trauma-exposed groups did not differ in terms of age, education, trauma history, or scores on the Clinician-Administered PTSD Scale, the trait version of the State-Trait Anxiety Inventory, the Dissociative Experiences Scale—Taxon, the Beck Depression Inventory II, or the Alcohol Use Disorders Identification Test. As can be seen, the trauma-exposed group reported higher Beck Anxiety Inventory scores at time 1 than the non-trauma-exposed group, and the mean number of days between time 1 and time 2 assessments was significantly greater in the non-trauma-exposed than the trauma-exposed group. Accordingly, an ANCOVA that entered assessment interval as a covariate was employed to analyze the time 2 psychometric data. Analyses indicated that the trauma-exposed group had higher scores than the non-trauma-exposed group on the Impact of Event Scale intrusion score, the Impact of Event Scale total score, the Peritraumatic Dissociative Experiences Questionnaire score, and the Acute Stress Disorder Interview score. ANCOVAs performed on time 2 Beck Anxiety Inventory scores, with control for both the group difference in assessment interval and time 1 Beck Anxiety Inventory scores, revealed no difference between the trauma-exposed and the non-trauma-exposed groups. At time 2, no subjects met criteria for acute stress disorder.
Resting Physiological Levels
Group mean resting physiological levels at time 1 and 2 are presented in Table 2. To assess the relationship between baseline physiological measures at times 1 and 2, a series of two-by-two (group-by-assessment) repeated-measures ANCOVAs were conducted separately for each dependent variable (skin conductance and EMG), with control for the difference between groups in assessment interval. There were no significant group or assessment main effects or group-by-assessment interactions for either physiological variable.
Physiological Responses to the Tones
Group mean physiological responses to the 15 tone trials at times 1 and 2 are presented in Figure 1 and Figure 2. Group mean physiological responses averaged across the 15 trials, slopes, and trials to nonresponse at times 1 and 2 are presented in Table 2. To assess the relationship between physiological responses at times 1 and 2, a series of two-by-two (group-by-assessment) repeated-measures ANCOVAs were conducted on mean response, response slope, and number of trials to nonresponse for EMG and skin conductance, with control for the difference between groups in assessment interval. Analyses revealed no significant group or assessment main effects or group-by-assessment interactions for any physiological variable.
Prediction of Posttrauma Psychophysiology
A series of stepwise multiple regression analyses were performed to identify the time 1 predictors of the time 2 psychophysiology measures. Pearson’s product-moment correlations, shown in Table 3, were calculated for the trauma-exposed group between selected time 2 psychophysiology-dependent variables and time 1 psychometric and psychophysiological variables to determine a subset of potential predictors for the regressions. To predict each time 2 psychophysiological measure, the psychometric and physiological time 1 variable that correlated the highest with the dependent variable was entered with assessment interval into the regression equation. This approach for restricting the battery of potential predictors for regression analyses has been employed in previous prospective research (42).
To predict time 2 baseline skin conductance level in the trauma-exposed group, time 1 resting skin conductance level, Dissociative Experiences Scale—Taxon score, and assessment interval were entered into the regression. Resting skin conductance level explained 12% of the variance in time 2 skin conductance level (β=0.38, SE=0.13, t=2.4, df=32, p<0.05), and Dissociative Experiences Scale—Taxon score accounted for a further 10% (β=–0.36, SE=0.02, t=–2.3, df=31, p<0.05). To predict time 2 skin conductance mean response, the time 1 variables entered into the regression equation were resting skin conductance level, Beck Depression Inventory II score, and the interval between assessments. Of these variables, resting skin conductance level accounted for 22% of the variance (β=0.50, SE=0.01, t=3.2, df=32, p<0.005). The subset of time 1 variables entered into the regression equation to predict time 2 number of skin conductance trials to nonresponse was trials to skin conductance nonresponse, years of education, and the interval between assessments. Time 1 number of trials to skin conductance nonresponse was the only significant predictor and explained 14% of the variance (β=0.41, SE=0.14, t=2.6, df=32, p<0.05).
To predict time 2 mean EMG response in the trauma-exposed group, the time 1 variables entered into the regression equation were mean EMG response, Alcohol Use Disorders Identification Test score, and the assessment interval. Of these variables, mean EMG response was the only significant predictor, explaining 61% of the variance (β=0.79, SE=0.11, t=7.3, df=32, p<0.001). To predict time 2 number of EMG trials to nonresponse, the time 1 variables entered into the regression were the number of EMG trials to nonresponse, prestartle anxiety rating, and the assessment interval. Time 1 number of trials to EMG nonresponse was the only significant predictor and accounted for 16% of the variance (β=0.43, SE=0.16, t=2.7, df=32, p<0.05).
An identical series of multiple regression analyses was performed for the non-trauma-exposed group, using the same predictors employed for the trauma-exposed group analyses. Time 1 number of trials to skin conductance nonresponse was the only significant predictor of time 2 trials to skin conductance nonresponse, explaining 18% of the variance (β=0.45, SE=0.15, t=3.0, df=34, p<0.01). The only significant predictor of time 2 mean EMG response was time 1 mean EMG response, which accounted for 44% of the variance (β=0.68, SE=0.13, t=5.4, df=34, p<0.001). In the non-trauma-exposed group, there were no significant predictors of time 2 resting skin conductance level, mean skin conductance response, or trials to EMG nonresponse from the time 1 variables entered into the regression equations.
Prediction of Posttrauma Symptom Profiles
Because of the absence of acute stress disorder cases, a dimensional approach was taken to assess the relationship between pretrauma variables and posttrauma psychopathology. A stepwise multiple regression analysis was performed to identify the time 1 predictors of time 2 Impact of Event Scale total score in the trauma-exposed group. Table 3 shows the Pearson product-moment correlations between the Impact of Event Scale score and the time 1 psychometric and psychophysiological variables in the trauma-exposed group. A subset of potential predictors was chosen for the regression, entering the psychometric and physiological time 1 variable that correlated the highest with time 2 Impact of Event Scale total score. The time 1 variables entered into the regression were mean skin conductance response, the score on the Alcohol Use Disorders Identification Test, and the assessment interval. Of these variables, the Alcohol Use Disorders Identification Test score accounted for 23% of the variance (β=0.48, SE=0.18, t=3.6, df=33, p<0.005), and mean skin conductance response accounted for a further 18% of the variance (β=0.42, SE=2.0, t=3.2, df=32, p<0.005). (With resting skin conductance level entered into the regression to control for baseline arousal, the Alcohol Use Disorders Identification Test score and skin conductance response for 15 trials remained the only significant predictors of Impact of Event Scale total score.) With the same variables entered into a stepwise multiple regression analysis for the non-trauma-exposed group as a validity check, there were no significant predictors of time 2 Impact of Event Scale score.
Discussion
The relationship between the time 1 and time 2 patterns of psychophysiological response to startle stimuli supports the hypothesis that pretrauma psychophysiological arousal may represent a vulnerability factor for heightened reactivity posttrauma. With regard to skin conductance responses, the current results support previous literature suggestive of a strong genetic component to electrodermal habituation (43). The larger eye blink EMG and skin conductance response magnitudes and slower habituation rates to startle stimuli reported previously in individuals with PTSD (3–8) may therefore reflect a pretrauma pattern of psychophysiological activity.
The results of the present study indicate that the magnitude of the pretrauma skin conductance response to startle stimuli was a positive predictor of posttraumatic stress symptoms in the acute trauma phase, as measured by the Impact of Event Scale score. These data lend support to the hypothesis that physiological reactivity evaluated pretrauma is a vulnerability factor for greater levels of distress in the acute trauma phase and to biological models emphasizing the role of acute arousal in the genesis of posttraumatic stress symptoms (15, 44–46). Previous findings of heightened arousal in the acute trauma phase in those who developed PTSD have been interpreted as reflecting a stronger unconditioned response (10). Heightened physiological reactivity may therefore be a vulnerability factor for stronger unconditioned response in the acute trauma phase, mediating fear conditioning that contributes to ongoing posttraumatic stress symptoms.
The relationship between pretrauma alcohol use and posttraumatic stress symptoms is consistent with previous findings of a shared genetic vulnerability between alcohol use and PTSD (19, 20). Despite the significant contribution of alcohol use, the magnitude of the pretrauma autonomic response to startle was still a strong predictor of posttrauma psychopathology in the present study. This highlights the importance of psychophysiological vulnerability to the development of posttraumatic stress symptoms. Vulnerability factors could be further indexed in future studies by an assessment of other factors, such as intelligence, given that previous research has demonstrated an association between lower intelligence and PTSD symptom severity (47).
It is possible that a negative response bias may have minimized symptom reporting in the trauma-exposed group. Previous research has identified lower rates of posttraumatic stress symptoms in trauma-exposed firefighters with high social desirability scores (48). Consistent with this finding, there was a negative correlation between Social Desirability Scale and Impact of Event Scale scores in the trauma-exposed group in this study.
These conclusions are limited by a number of factors. First, the lack of acute stress disorder cases in this trauma-exposed group necessitated a focus on posttraumatic stress symptom severity rather than diagnosis. Second, we recognize that the group is small, and the study needs to be replicated with a larger cohort. Third, although the current data sheds light on predictors of acute stress response, it does not permit inferences about chronic stress reactions. Longer-term follow-up assessments are required to discriminate between predictors of transient stress response and early posttraumatic stress symptoms that are precursors to chronic PTSD. Fourth, firefighters may represent a specific population, and the conclusions may not necessarily be generalized to other trauma-exposed populations. Despite these limitations, the present data offer some preliminary evidence for the role of pretrauma arousal in the development of posttraumatic stress symptoms after trauma exposure. Evidence of a predisposition toward heightened reactivity before trauma exposure offers a promising avenue for future research into understanding the biological mechanisms of posttraumatic stress reactions.
 |
 |
 |
Received Dec. 1, 2003; revision received Jan. 23, 2004; accepted April 9, 2004. From the School of Psychology, University of New South Wales. Address correspondence and reprint requests to Dr. Bryant, School of Psychology, University of New South Wales, Sydney, N.S.W. 2052 Australia; [email protected] (e-mail). The authors thank the New South Wales Fire Brigades for agreeing to participate in the study and the participating firefighters. Funded by National Health and Medical Research Council Program Grant (300304) and an Australian postgraduate award.
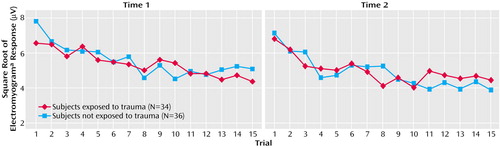
Figure 1. Mean Physiological Responses of Firefighters to 15 Tones at Time 1 and Time 2
aElectromyogram of left orbicularis oculi.
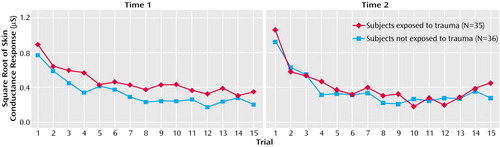
Figure 2. Mean Physiological Responses of Firefighters to 15 Tones at Time 1 and Time 2
1. Pitman RK, Orr SP: Psychophysiologic testing for post-traumatic stress disorder: forensic psychiatric application. Bull Am Acad Psychiatry Law 1993; 21:37–52Medline, Google Scholar
2. Yehuda R: Sensitization of the hypothalamic-pituitary-adrenal axis in posttraumatic stress disorder, in Psychobiology of Posttraumatic Stress Disorder. Edited by Yehuda R, McFarlane A. New York, New York Academy of Sciences, 1997, pp 57–75Google Scholar
3. Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA: Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. Am J Psychiatry 1990; 147:1308–1312Link, Google Scholar
4. Shalev AY, Orr SP, Peri T, Schreiber S, Pitman RK: Physiologic responses to loud tones in Israeli patients with posttraumatic stress disorder. Arch Gen Psychiatry 1992; 49:870–875Crossref, Medline, Google Scholar
5. Orr SP, Lasko NB, Shalev AY, Pitman RK: Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J Abnorm Psychol 1995; 104:75–82Crossref, Medline, Google Scholar
6. Shalev AY, Peri T, Orr SP, Bonne O, Pitman RK: Auditory startle responses in help-seeking trauma survivors. Psychiatry Res 1997; 69:1–7Crossref, Medline, Google Scholar
7. Metzger LJ, Orr SP, Berry NJ, Ahern CE, Lasko NB, Pitman RK: Physiologic reactivity to startling tones in women with posttraumatic stress disorder. J Abnorm Psychol 1999; 108:347–352Crossref, Medline, Google Scholar
8. Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, Pitman RK: Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. Am J Psychiatry 2000; 157:255–261Link, Google Scholar
9. Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M: Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry 1993; 50:294–305Crossref, Google Scholar
10. Shalev AY, Sahar T, Freedman S, Peri T, Glick N, Brandes D, Orr SP, Pitman RK: A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Arch Gen Psychiatry 1998; 55:553–559Crossref, Medline, Google Scholar
11. Bryant RA, Harvey AG, Guthrie RM, Moulds ML: A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. J Abnorm Psychol 2000; 109:341–344Crossref, Medline, Google Scholar
12. Blanchard EB, Hickling EJ, Galovski T, Veazey C: Emergency room vital signs and PTSD in a treatment seeking sample of motor vehicle accident survivors. J Trauma Stress 2002; 15:199–204Crossref, Medline, Google Scholar
13. McFarlane A, Atchison M, Yehuda R: The acute stress response following motor vehicle accidents and its relation to PTSD, in Psychobiology of Posttraumatic Stress Disorder. Edited by Yehuda R, McFarlane A. New York, New York Academy of Sciences, 1997, pp 437–441Google Scholar
14. Resnick HS, Yehuda R, Pitman RK, Foy DW: Effect of previous trauma on acute plasma cortisol level following rape. Am J Psychiatry 1995; 152:1675–1677Link, Google Scholar
15. Post RM, Weiss SR, Smith MA: Sensitization and kindling: implications for the evolving neural substrates of post-traumatic stress disorder, in Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to Post-Traumatic Stress Disorder. Edited by Friedman MJ, Charney DS, Deutch AY. Philadelphia, Lippincott Williams & Wilkins, 1995, pp 203–224Google Scholar
16. Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK: Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with posttraumatic stress disorder. Arch Gen Psychiatry 2003; 60:283–288Crossref, Medline, Google Scholar
17. Shalev AY, Rogel-Fuchs Y: Psychophysiology of the posttraumatic stress disorder: from sulfur fumes to behavioral genetics. Psychosom Med 1993; 55:413–423Crossref, Medline, Google Scholar
18. Brewin CR, Andrews B, Valentine JD: Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol 2000; 68:748–766Crossref, Medline, Google Scholar
19. Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR: Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend 2000; 61:95–102Crossref, Medline, Google Scholar
20. McLeod DS, Koenen KC, Meyer JM, Lyons MJ, Eisen S, True W, Goldberg J: Genetic and environmental influences on the relationship among combat exposure, posttraumatic stress disorder symptoms, and alcohol use. J Trauma Stress 2001; 14:259–275Crossref, Medline, Google Scholar
21. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
22. Blake DD, Weathers FW, Nagy LN, Kaloupek DG, Klauminzer G, Charney DS, Keane TM: A clinician rating scale for assessing current and lifetime PTSD: the CAPS. 1. Behavior Therapist 1990; 18:187–188Google Scholar
23. Weathers FW, Litz BL: Psychometric properties of the Clinician-Administered PTSD Scale, CAPS-1. PTSD Res Quarterly 1994; 5:2–6Google Scholar
24. Vrana S, Lauterbach D: Prevalence of traumatic events and post-traumatic psychological symptoms in a nonclinical sample of college students. J Trauma Stress 1994; 7:289–302Crossref, Medline, Google Scholar
25. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA: Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
26. Beck AT, Steer RA: Manual for the Beck Anxiety Inventory. San Antonio, Tex, Psychological Corp, 1990Google Scholar
27. Beck AT, Steer RA, Brown GK: Manual for the Beck Depression Inventory II. San Antonio, Tex, Psychological Corp, 1996Google Scholar
28. Waller N, Putnam FW, Carlson EB: Types of dissociation and dissociative types: a taxometric analysis of dissociative experiences. Psychol Methods 1996; 1:300–321Crossref, Google Scholar
29. Bernstein EM, Putnam FW: Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 1986; 174:727–735Crossref, Medline, Google Scholar
30. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M: Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption: II. Addiction 1993; 88:791–804Crossref, Medline, Google Scholar
31. Bryant RA, Harvey AG, Dang ST, Sackville T: Assessing acute stress disorder: psychometric properties of a structured clinical interview. Psychol Assess 1998; 10:215–220Crossref, Google Scholar
32. Horowitz MJ, Wilner N, Alvarez W: Impact of Event Scale: a measure of subjective stress. Psychosom Med 1979; 41:209–218Crossref, Medline, Google Scholar
33. Marmar CR, Weiss DS, Metzler TJ: The Peritraumatic Dissociative Experiences Questionnaire, in Assessing Psychological Trauma and PTSD. Edited by Wilson JP, Keane TM. New York, Guilford, 1997, pp 412–428Google Scholar
34. Crowne DP, Marlow D: A new scale of social desirability independent of psychopathology. J Consult Clin Psychol 1960; 24:349–354Crossref, Google Scholar
35. Fridlund AJ, Cacioppo JT: Guidelines for human electromyographic research. Psychophysiology 1986; 23:567–589Crossref, Medline, Google Scholar
36. Fowles DC: Publication recommendations for electrodermal measurements. Psychophysiology 1981; 18:232–239Crossref, Medline, Google Scholar
37. Venables PH, Christie MJ: Mechanisms, instrumentation, recording techniques and quantification of responses, in Electrodermal Activity in Psychological Research. Edited by Prokasy WF, Raskin DC. New York, Academic Press, 1973, pp 1–124Google Scholar
38. Stern RM, Ray WJ, Quigley KS: Psychophysiological Recording, 2nd ed. New York, Oxford University Press, 2001Google Scholar
39. Bradley MM, Cuthbert BN, Lang PJ: Startle reflex modification: emotion or attention? Psychophysiology 1990; 27:513–522Crossref, Medline, Google Scholar
40. Berg WK, Balaban MT: Startle elicitation: stimulus parameters, recording techniques, and quantification, in Startle Modification: Implications for Neuroscience, Cognitive Science, and Clinical Science. Edited by Dawson ME, Schell AM, Bohmelt AH. New York, Cambridge University Press, 1999, pp 21–50Google Scholar
41. Larson CL, Ruffalo D, Nietert JY, Davidson RJ: Temporal stability of the emotion-modulated startle response. Psychophysiology 2000; 37:92–101Crossref, Medline, Google Scholar
42. Blanchard EB, Hickling EJ, Barton KA, Taylor AE, Loos WR, Jones-Alexander J: One-year prospective follow-up of motor vehicle accident victims. Behav Res Ther 1996; 34:775–786Crossref, Medline, Google Scholar
43. Lykken DT, Iacono WG, Haroian K, McGue M: Habituation of the skin conductance response to strong stimuli: a twin study. Psychophysiology 1988; 25:4–15Crossref, Medline, Google Scholar
44. Kolb LC: A neuropsychological hypothesis explaining posttraumatic stress disorders. Am J Psychiatry 1987; 144:989–995Link, Google Scholar
45. Pitman RK: Post-traumatic stress disorder, hormones, and memory. Biol Psychiatry 1989; 26:221–223Crossref, Medline, Google Scholar
46. van der Kolk BA: The body keeps score: approaches to the psychobiology of posttraumatic stress disorder, in Traumatic Stress: The Effects of Overwhelming Experience on Mind, Body, and Society. Edited by van der Kolk BA, McFarlane AC, Weisaeth L. New York, Guilford, 1996, pp 214–241Google Scholar
47. McNally RJ, Shin LM: Association of intelligence with severity of posttraumatic stress disorder symptoms in Vietnam combat veterans. Am J Psychiatry 1995; 152:936–938Link, Google Scholar
48. Wagner D, Heinrichs M, Ehlert U: Prevalence of symptoms of posttraumatic stress disorder in German professional firefighters. Am J Psychiatry 1998; 155:1727–1732Link, Google Scholar



