Lithium Placental Passage and Obstetrical Outcome: Implications for Clinical Management During Late Pregnancy
Abstract
OBJECTIVE: Lithium has been used during pregnancy for more than four decades, but quantification of fetal lithium exposure and clinical correlations of such exposure are limited. The study objectives were to 1) quantify the rate of lithium placental passage, 2) assess any association between plasma concentration of lithium at delivery and adverse perinatal events, and 3) determine whether lithium concentrations can be reduced by briefly suspending therapy proximate to delivery. METHOD: Maternal blood and umbilical cord blood were obtained at delivery for assay of lithium concentrations, and obstetrical outcome data were collected prospectively for 10 participants. These data were combined with results from MEDLINE and PsycINFO searches that identified 32 cases in which maternal lithium was administered throughout delivery. Statistical analysis of the pooled data was conducted. RESULTS: The ratio of lithium concentrations in umbilical cord blood to maternal blood (mean=1.05, SD=0.13) was uniform across a wide range of maternal concentrations (0.2–2.6 meq/liter). Significantly lower Apgar scores, longer hospital stays, and higher rates of CNS and neuromuscular complications were observed in infants with higher lithium concentrations (>0.64 meq/liter) at delivery. Withholding lithium therapy for 24–48 hours before delivery resulted in a 0.28 meq/liter reduction in maternal lithium concentration. CONCLUSIONS: Lithium completely equilibrates across the placenta. Higher lithium concentrations at delivery are associated with more perinatal complications, and lithium concentrations can be reduced by brief suspension of therapy proximate to delivery. Treatment guidelines are proposed to improve neonatal well-being when lithium use is indicated in late pregnancy.
The management of bipolar disorder during pregnancy remains one of the most daunting challenges of psychiatric practice. Although the risk for relapse of bipolar disorder during gestation is poorly characterized, and evidence is conflicting as to whether pregnancy alters such risk favorably or unfavorably (1–13), several studies have indicated that, in the absence of continued pharmacotherapy, 50%–60% of women with bipolar disorder will relapse during pregnancy (2, 14, 15). Relapse rates are higher after abrupt cessation of lithium therapy, and relapse rates have been poorly evaluated after abrupt discontinuation of other mood stabilizers (2). Postpartum recurrence of bipolar disorder is especially common (2–4, 12, 16–21), and postpartum psychosis is more common among women with bipolar disorder, demonstrating a 100-fold increase over the background rate of 0.05% (22–24). This vulnerability to perinatal relapse, coupled with evidence that maternal psychiatric illness carries untoward risks for fetal development (25–27), underscores the need for effective clinical management during gestation.
There are, however, numerous reproductive safety concerns regarding the medications used to treat bipolar disorder. Psychotropic agents used to manage bipolar disorder are either known teratogens or have limited reproductive safety data that preclude sound risk estimates (27). Since the 1950s, lithium has been the cornerstone of pharmacotherapy for bipolar disorder. Its use in pregnancy has garnered considerable attention. Initial retrospective analyses suggested that lithium exposure was associated with a 400-fold increase in the rate of Ebstein’s anomaly, a tricuspid valve malformation (28, 29), but subsequent meta-analyses indicated that the risk ratio for cardiac malformations after lithium exposure is only 1.2 to 7.7 (30) and the risk for Ebstein’s anomaly rises from 1 in 20,000 to 1 in 1,000 (31). It has been argued, however, that these revised estimates could be low as an artifact of study methods, because Ebstein’s anomaly may have been underrecognized in previous reports as a result of the inconsistent use of fetal echocardiography (32). These revised estimates of lithium teratogenicity are considerably lower than estimates of the teratogenic risks of valproate and carbamazepine (27). Furthermore, lithium is the only mood stabilizer for which published neurodevelopmental outcome data show a lack of adverse effects among prenatally exposed children (33). Early findings from a lamotrigine pregnancy registry are promising, compared to findings for other anticonvulsants (34), but additional cases and infant follow-up studies are needed.
Although lithium is not devoid of prenatal safety concerns, and its use during lactation has been widely discouraged (35), it continues to be favored by many clinicians for use during pregnancy, given its favorable safety profile relative to other mood stabilizers. Nevertheless, lithium use during late pregnancy is associated with particular clinical concerns that have not been systematically investigated, and the literature includes numerous reports of neonatal complications in association with lithium treatment during late pregnancy, including cardiac dysfunction (36–47), diabetes insipidus (37, 38, 48, 49), hypothyroidism (37, 48, 49), low muscle tone (36–40, 42–44, 46, 48, 50, 51), lethargy (36, 48, 52, 53), hepatic abnormalities (36–39, 42, 45, 46), and respiratory difficulties (36–40, 42–46, 48). Recognizing lithium’s low therapeutic index, it seems plausible that such complications are related to the level of lithium exposure proximate to delivery, although some reviewers contend that the occurrence of such effects is independent of lithium concentration (38, 42).
The objective of the present study was to address this deficiency in lithium’s reproductive safety data by 1) extending data quantifying the rate of lithium placental passage, 2) testing for an association between infant lithium concentration at delivery and the incidence of perinatal adverse events, and 3) determining whether lithium concentrations can be reduced by briefly suspending treatment immediately before delivery.
Method
Subjects
Participants were recruited from patients who were referred to the Emory Women’s Mental Health Program for the perinatal management of bipolar disorder and were enrolled in a prospective observational study of the clinical course of bipolar disorder during pregnancy and the postpartum period. Subjects were informed of the risks associated with fetal and infant exposure to lithium as well as the risks of untreated maternal bipolar disorder. In addition, the risks and benefits of alternative treatment options, including other mood stabilizers, psychotherapy, and electroconvulsive therapy, were reviewed. In an effort to reduce the potential for lithium-associated complications at delivery, Women’s Mental Health Program patients are empirically advised to suspend lithium administration 1–2 days before a scheduled delivery or at the onset of labor in the event of spontaneous delivery. Dose suspension is a standard of care at the Women’s Mental Health Program clinic that was not randomly assigned or in any respect dictated by study participation. Data are presented for 10 study participants who elected to continue lithium therapy during late pregnancy. Each participant fulfilled the DSM-IV criteria for bipolar I disorder or bipolar II disorder. The Institutional Review Board of the Emory University School of Medicine approved the study.
Data Collection
Study data included prospective documentation of prenatal psychiatric treatment, obstetrical outcome as recorded on the Women’s Mental Health Program Delivery Information Sheet and the hospital delivery record, and assays of maternal and umbilical cord blood samples collected during gestation and at delivery. Blood samples were collected in chilled EDTA-containing tubes, placed on ice, and centrifuged at 4°C for 10 minutes at 3,000 rpm. Plasma was coded and stored at –80°C until assay.
Laboratory personnel who were blind to the subjects’ demographic characteristics and to the source of the blood samples conducted lithium assays with an ion-selective electrode instrument (Beckman Coulter, Inc., Fullerton, Calif.).
Study data were extended by incorporating findings from previous reports of fetal and maternal lithium concentrations and obstetrical outcome among women taking lithium at delivery. The previous reports were identified through a search of the English-language literature by using MEDLINE and PsycINFO databases.
Data Analysis
A descriptive analysis was performed to characterize the placental passage of lithium. The ratio of lithium concentration in infant (umbilical cord) plasma to that in maternal plasma was calculated for each maternal-infant pair as an index of lithium placental passage, and a scatterplot was constructed of the calculated ratios. This analysis was performed by using data for each dyad from the Women’s Mental Health Program participants and data from previous published studies that reported both infant and maternal lithium concentrations.
The hypothesis that higher infant lithium concentrations at delivery are associated with increased vulnerability to perinatal complications was tested by using data for the infants in the Women’s Mental Health Program group and data from previous studies that reported both infant lithium concentrations at delivery and obstetrical outcomes. The median neonatal lithium concentration was calculated, and infants whose lithium concentration at delivery was greater than the median were assigned to the high lithium exposure group. The remaining infants were assigned to the low lithium exposure group. Hypothesis testing to assess group differences with respect to various complications was performed by using frequency tests (Fisher’s exact test) for nominal variables and one-sample t tests for continuous variables.
Finally, the hypothesis that lithium concentrations at delivery could be significantly reduced by suspending treatment within 48 hours of anticipated delivery was tested by using a paired t test of maternal lithium concentrations at delivery and earlier measures during pregnancy for the participants from the Women’s Mental Health Program. All statistical tests were two-tailed.
At relevant points in the analysis, findings from the Women’s Mental Health Program group were compared to those from previous reports to assess the validity of the pooled analysis.
Results
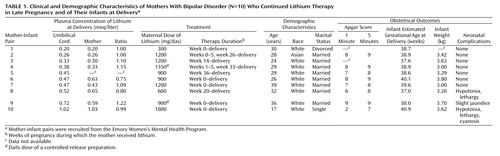
The demographic and clinical characteristics of the Women’s Mental Health Program participants are presented in Table 1. Lithium concentrations were determined for the 10 umbilical cord samples and nine of the 10 maternal samples (one sample was lost). In addition to the Women’s Mental Health Program participants, 32 fetal-maternal pairs exposed to lithium at delivery were identified from published reports (36–60). Lithium concentrations were reported for 18 of these 32 dyads (38–40, 48, 52, 53, 55–58).
The mean infant-mother lithium ratio at delivery for the 27 pairs was 1.05 (SD=0.13). A scatterplot of the ratios is depicted in Figure 1. For these 27 pairs, the mean infant lithium concentration at delivery was 0.72 meq/liter (SD=0.57, range=0.20–2.80), and the mean maternal concentration was 0.71 meq/liter (SD=0.59, range=0.20–2.60). Although infant (0.49 versus 0.84 meq/liter) and maternal (0.49 versus 0.82 meq/liter) concentrations were lower in the Women’s Mental Health Program group than in the infant-mother pairs from previous reports, infant-mother lithium ratios were remarkably similar (1.01 versus 1.06) between the two samples. Despite the lower lithium concentrations at delivery, daily lithium doses in the Women’s Mental Health Program mothers (mean=1050 mg/day, SD=437) were marginally higher than those in the mothers described in earlier publications (mean=1013 mg/day, SD=493).
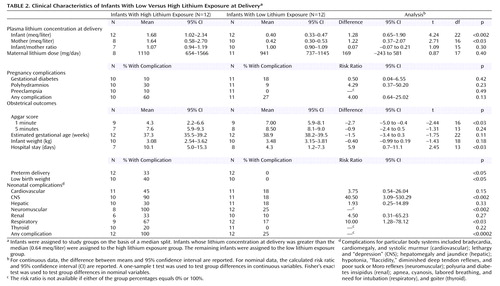
Lithium concentrations and obstetrical outcomes were available for the 10 infants in the Women’s Mental Health Program group and for 14 of the 32 infants described in earlier reports (36, 38–40, 42, 44, 48, 49, 51, 52, 53, 56, 57, 59). In the resulting group of 24 infants, 12 with lithium concentrations below the median of 0.64 meq/liter were assigned to the low lithium exposure group (range=0.20–0.58 meq/liter), and 12 with lithium concentrations above the median were assigned to the high lithium exposure group (range=0.70 to >4.0 meq/liter). The results of hypothesis testing are presented in Table 2. The mean infant-mother lithium ratio at delivery was virtually identical between the low lithium exposure and high lithium exposure groups, indicating that the degree of placental passage does not vary with maternal plasma lithium concentration within the clinical range of concentrations encountered.
The rate of all complications, with the exception of gestational diabetes, was consistently higher in the high lithium exposure group. In particular, the rates of CNS and neuromuscular complications were significantly higher, the duration of infant hospital stays was significantly longer, and 1-minute Apgar scores were significantly lower in the high lithium exposure group. The rates of preterm delivery, low birth weight, and infant respiratory complications were higher in the high lithium exposure group than in the low lithium exposure group, but the differences only approached significance. Even when the data for five infants in the high lithium exposure group who had lithium concentrations at delivery in the “toxic” range (>1.2 meq/liter) were omitted from the analysis, the rates of CNS complications (83%) (p<0.04, Fisher’s exact test) and neuromuscular complications (100%) (p<0.01, Fisher’s exact test) remained significantly higher in the high lithium exposure group, and this group had longer infant hospital stays (mean=10.0 days) than the low lithium exposure group, although the difference only approached significance. Because the Women’s Mental Health Program participants had been instructed to suspend lithium treatment before delivery, these participants’ infants are overrepresented in the low lithium exposure group (eight of 12), compared to the high lithium exposure group (two of 12), and had a lower rate of complications than the mother-infant pairs from previous reports.
Although maternal lithium concentrations at delivery were subtherapeutic (<0.5 meq/liter) for five of the nine women in the Women’s Mental Health Program group (mean=0.49 meq/liter, SD=0.26, range=0.20–1.03), they were in the therapeutic range for all Women’s Mental Health Program participants when sampled at the most recent prenatal visit (mean=0.79 meq/liter, SD=0.19, range=0.50–1.10). A paired t test indicated that maternal lithium concentrations at delivery were significantly lower than those during pregnancy (mean difference=0.28 meq/liter, 95% confidence interval [CI]=0.08–0.49; t=3.38, df=6, p<0.02), with a mean of 20.6 hours (SD=19.2) having elapsed since the last lithium dose when the delivery sample was collected. Despite the brief interruption in lithium therapy, only one of the 10 Women’s Mental Health Program participants was symptomatic in the perinatal period. That patient experienced a depressive episode with psychotic features that began 2 weeks before delivery, prior to any suspension of lithium therapy.
Discussion
To our knowledge, this study is the first to examine systematically the clinical relevance of lithium placental passage at delivery. The study’s findings are as follows:
| 1. | Lithium demonstrates complete placental passage as evidenced by the mean infant-mother lithium ratio of 1.05. | ||||
| 2. | Lithium ion equilibration across the placental barrier is remarkably uniform across a wide range of maternal concentrations (0.2–2.6 meq/liter), suggesting that lithium equilibrates between maternal and fetal circulation much as it does across the total body water space in an individual. This finding is consistent with the results of preclinical studies demonstrating minimal transplacental differences in electric potential after acute lithium administration (60, 61). | ||||
| 3. | Adverse perinatal outcomes are more extensive in the setting of higher lithium concentrations at delivery as evidenced by the consistently higher rate of varied complications among infants in the high lithium exposure group. | ||||
| 4. | Lithium delivery concentrations can be significantly reduced at delivery without compromising pharmacotherapeutic efficacy by briefly withholding lithium therapy. | ||||
This constellation of findings bears important implications for treatment guidelines, now woefully inadequate, when lithium administration is indicated during late gestation. Conflicting recommendations have been proposed regarding lithium’s use in late pregnancy, with some experts urging lithium dose reduction or discontinuation long before delivery to avoid neonatal toxicity (36, 47) and others advocating the reinstitution of lithium therapy before delivery to avoid the high risk of postpartum relapse in patients with bipolar disorder (62). Moreover, despite its use for more than five decades, no guidelines have been established for therapeutic monitoring of lithium concentrations during pregnancy or potential adverse effects beyond surveillance for cardiac teratogenicity (63). Because poor outcomes in this study were predicted by higher infant lithium concentrations at delivery, and neonate concentrations invariably approximated maternal concentrations, infant well-being can be enhanced by monitoring maternal lithium concentrations as delivery approaches. Furthermore, the ability to lower maternal concentrations expeditiously by suspending lithium therapy for 24–48 hours before delivery might improve obstetrical outcome as neonatal concentrations can be reduced without unduly compromising treatment efficacy.
Neonatal effects of lithium exposure are of gravest concern when maternal toxicity arises before delivery. Although lithium toxicity did not complicate the pregnancies of the Women’s Mental Health Program participants, earlier reports indicate that maternal toxicity during late gestation warrants concern. Of seven reported cases of prenatal lithium toxicity (39, 44, 46, 50, 52–54), the etiology is identifiable in only three. In two cases, toxicity arose iatrogenically after the institution of diuretic therapy and a sodium-restricted diet to manage edema (44, 54). Toxicity occurred in another case when a sodium-restricted diet was initiated to manage preeclampsia (52), a condition that decreases the glomerular filtration rate (64–66) and, ostensibly, lithium clearance. The cause of lithium toxicity in the remaining cases (39, 46, 49, 50) is unclear. Toxicity may have been a consequence of intentional or accidental overdose, although this cause was not explicitly stated, and pregnancy-specific causes, such as abrupt fluid loss during labor, cannot be eliminated.
From these findings, we propose the following guidelines for lithium administration during late pregnancy:
| 1. | Maintain a target lithium concentration at the minimum effective level for the individual. Although lithium levels of 0.8–1.0 meq/liter are widely cited as the ideal for maintenance therapy, some individuals can be successfully maintained at lower concentrations (67). | ||||
| 2. | Periodically monitor lithium concentrations during pregnancy, particularly during late gestation when marked changes in glomerular filtration rate can alter lithium clearance (66, 68, 69). | ||||
| 3. | Avoid pregnancy treatment modalities that increase the potential for lithium toxicity (Table 3). However, if such measures are obstetrically warranted, more frequent lithium monitoring and dose reduction are indicated. | ||||
| 4. | Lower maternal lithium dose in the event of pregnancy complications such as preeclampsia (64–66) or polyhydramnios (38, 39, 47, 48) (Table 3) that might predispose the patient or her child to lithium toxicity. Similarly, any abnormality in amniotic fluid volume, including oligohydramnios, could be the result of and/or could increase the likelihood of lithium-associated fetal nephrotoxicity. | ||||
| 5. | Suspend lithium administration 24–48 hours before a scheduled Cesarean section or induction, or suspend lithium administration at the onset of labor in the event of spontaneous delivery. | ||||
| 6. | Check the maternal lithium concentration at the patient’s presentation to the hospital for delivery. | ||||
| 7. | Administer oral and/or intravenous fluids throughout the labor and delivery process, and check maternal lithium concentration in the event of clinical signs of toxicity. | ||||
| 8. | Reinstitute lithium therapy as soon as the patient is medically stabilized after delivery. Use the preconception dose, because the maternal glomerular filtration rate rapidly returns to pregravid levels after delivery. | ||||
This study is not without shortcomings. The small number of participants limited the statistical power to elucidate fully the association between clinical outcome and lithium concentration. Another limitation is the demographic homogeneity of the 10 Women’s Mental Health Program participants, which limits the generalizability of the study results. It can also be argued that the dependability of the study findings is weakened by the inclusion of case data from earlier reports that in some instances were incomplete. Conversely, the consistent character of the findings when the data are analyzed across so many reports, despite probable differences in data collection, serves to reinforce the reliability and potential generalizability of the findings.
In summary, given the considerable morbidity of untreated bipolar disorder and the significant risk of perinatal relapse in the absence of continued pharmacotherapy, prolonged discontinuation of treatment is seldom a viable option. Despite documented concerns about effects of lithium in reproduction, this medication remains the preferred alternative during gestation for many women with bipolar disorder. When lithium is used during late pregnancy, infant and maternal well-being can be maximized by maintaining maternal concentrations at the minimal effective level, suspending lithium therapy 24–48 hours before delivery to reduce neonatal concentrations further, and avoiding inadvertent or iatrogenic lithium toxicity during gestation.
 |
 |
 |
Received April 27, 2004; revision received Sept. 5, 2004; accepted Sept. 10, 2004. From the Departments of Psychiatry and Behavioral Sciences, Pharmacology, and Gynecology and Obstetrics, Emory University School of Medicine; and the Department of Psychiatry, Harvard Medical School, Boston. Address correspondence and reprint requests to Dr. Newport, Women’s Mental Health Program, Emory University School of Medicine, 1365 Clifton Rd., N.E., Suite B6100, Atlanta, GA 30322; [email protected] (e-mail). Supported in part by an NIH Patient-Oriented Research Career Development Award (MH-63507) and an NIH Specialized Center of Research grant (P50 MH-68036).
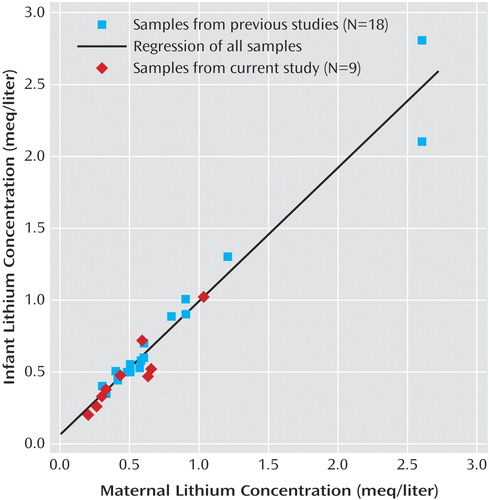
Figure 1. Concentrations of Lithium in Plasma Samples From Mothers With Bipolar Disorder Who Continued Lithium Therapy in Late Pregnancy and From Their Infants at Deliverya
aData are from mother-infant pairs recruited from the Emory Women’s Mental Health Program (current study) and from mother-infant pairs described in the literature (38–40, 48, 52, 53, 55–58).
1. Viguera AC, Baldessarini RJ, Hegarty JM, van Kammen D, Tohen M: Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry 1997; 54:49–55Crossref, Medline, Google Scholar
2. Viguera AC, Nonacs R, Cohen LS, Tondo L, Murray A, Baldessarini RJ: Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry 2000; 157:179–184Link, Google Scholar
3. Kendall RE, Chalmers JC, Platz C: Epidemiology of puerperal psychoses. Br J Psychiatry 1987; 150:662–673Crossref, Medline, Google Scholar
4. Freeman MP, Smith KW, Freeman SA, McElroy SL, Kmetz GE, Wright R, Keck PE: The impact of reproductive events on the course of bipolar disorder in women. J Clin Psychiatry 2000; 63:284–287Crossref, Google Scholar
5. Targum SD, Davenport YB, Webster MJ: Postpartum mania in bipolar manic depressive patients withdrawn from lithium carbonate. J Nerv Ment Dis 1979; 167:572–574Crossref, Medline, Google Scholar
6. McNeil TF, Kaij L, Malmquist-Larsson A: Women and nonorganic psychosis: factors associated with pregnancy’s effect on mental health. Acta Psychiatr Scand 1984; 70:209–219Crossref, Medline, Google Scholar
7. Sharma V, Persad P: Effect of pregnancy on three patients with bipolar disorder. Ann Clin Psychiatry 1995; 7:39–42Crossref, Medline, Google Scholar
8. Marzuk PM, Tardiff K, Leon AC, Hirsch CS, Portera L, Hartwell N, Iqbal MI: Lower risk of suicide during pregnancy. Am J Psychiatry 1997; 154:122–123Link, Google Scholar
9. Lier L, Kastrup M, Rafaelsen O: Psychiatric illness in relation to pregnancy and childbirth: diagnostic profiles, psychosocial and perinatal aspects. Nord Psykiatr Tidsskr 1989; 43:535–542Crossref, Google Scholar
10. Pugh TF, Schmidt WM, Reed RB: Rates of mental disease related to childbearing. N Engl J Med 1963; 268:1224–1228Crossref, Medline, Google Scholar
11. Nott PN: Psychiatric illness following childbirth in South Hampton: a case register study. Psychol Med 1982; 12:557–561Crossref, Medline, Google Scholar
12. Blehar MC: Gender differences in risk factors for mood and anxiety disorders: implications for clinical treatment research. Psychopharmacol Bull 1995; 31:687–691Medline, Google Scholar
13. Grof PR, Robbins W, Alda M, Berghoefer A, Vojtechovsky M, Nilsson A, Robertson C: Protective effect of pregnancy in women with lithium-responsive bipolar disorder. J Affect Disord 2000; 61:31–39Crossref, Medline, Google Scholar
14. Blehar M, Depaulo J, Gershon E, Reich T, Simpson SG, Nurnberger JI: Women with bipolar disorder: findings from the NIMH Genetics Initiative Sample. Psychopharmacol Bull 1988; 34:239–243Google Scholar
15. Finnerty M, Levin Z, Miller LJ: Acute manic episodes in pregnancy (case conf). Am J Psychiatry 1996; 153:261–263Link, Google Scholar
16. Bratfos O, Haug JO: Puerperal mental disorders in manic-depressive females. Acta Psychiatr Scand 1966; 42:285–294Crossref, Medline, Google Scholar
17. Reich T: Postpartum psychoses in patients with manic-depressive disease. J Nerv Ment Dis 1970; 151:60–68Crossref, Medline, Google Scholar
18. Brockington IF, Cernik KF, Schofield EM, Downing AR, Francis AF, Keelan C: Puerperal psychosis: phenomena and diagnosis. Arch Gen Psychiatry 1981; 38:829–833Crossref, Medline, Google Scholar
19. Davidson J, Robertson E: A follow-up study of postpartum illness. Acta Psychiatr Scand 1985; 71:451–457Crossref, Medline, Google Scholar
20. Klompenhouwer J, van Hulst A: Classification of postpartum psychosis: a study of 250 mother and baby admissions in the Netherlands. Acta Psychiatr Scand 1991; 84:255–261Crossref, Medline, Google Scholar
21. Rhode A: Postpartum psychosis: onset and long-term course. Psychopathology 1993; 26:203–209Crossref, Medline, Google Scholar
22. Brockington IF: The course and outcome of cycloid psychoses. Psychol Med 1982; 12:97–105Crossref, Medline, Google Scholar
23. Kendall RE: Emotional and physical factors in the genesis of puerperal mental disorders. J Psychosom Res 1985; 29:3–11Crossref, Medline, Google Scholar
24. Stewart DE, Klompenhouwer JL, Kendall RE, van Hulst AM: Prophylactic lithium in puerperal psychosis: the experience of three centers. Br J Psychiatry 1991; 158:393–397Crossref, Medline, Google Scholar
25. Stowe ZN, Calhoun K, Ramsey C, Sadek N, Newport DJ: Mood disorders during pregnancy and lactation: defining issues of exposure and treatment. CNS Spectr 2001; 6:150–166Crossref, Google Scholar
26. Newport DJ, Stowe ZN, Nemeroff CB: Parental depression: animal models of an adverse life event. Am J Psychiatry 2002; 159:1265–1283Link, Google Scholar
27. Newport DJ, Fisher A, Graybeal S, Stowe ZN: Psychopharmacology during pregnancy and lactation, in The American Psychiatric Publishing Textbook of Psychopharmacology. Edited by Schatzberg AF, Nemeroff CB. Washington, DC, American Psychiatric Publishing, 2004, pp 1109–1146Google Scholar
28. Nora JJ, Nora AH, Toews WH: Lithium, Ebstein’s anomaly, and other congenital heart defects. Lancet 1974; 2:594–595Crossref, Medline, Google Scholar
29. Weinstein MR, Goldfield M: Cardiovascular malformations with lithium use during pregnancy. Am J Psychiatry 1975; 132:529–531Link, Google Scholar
30. Cohen L, Friedman J, Jefferson J, Johnson E, Weiner M: A reevaluation of risk of in utero exposure to lithium. JAMA 1994; 271:146–150Crossref, Medline, Google Scholar
31. Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J: Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153:592–606Link, Google Scholar
32. Warner JP: Evidence-based psychopharmacology, 3: assessing evidence of harm: what are the teratogenic effects of lithium carbonate? J Psychopharmacol 2000; 14:77–80Crossref, Medline, Google Scholar
33. Schou M: What happened later to the lithium babies? follow-up study of children born without malformations. Acta Psychiatr Scand 1976; 54:193–197Crossref, Medline, Google Scholar
34. Cunnington M, Tennis P, International Lamotrigine Pregnancy Registry Scientific Advisory Committee: Lamotrigine and the risk of malformations in pregnancy. Neurology 2005; 64:995–960Crossref, Google Scholar
35. American Academy of Pediatrics Committee on Drugs: The transfer of drugs and other chemicals into human milk. Pediatrics 2001; 108:776–789Crossref, Medline, Google Scholar
36. Arnon RG, Marin-Garcia J, Peeden JN: Tricuspid valve regurgitation and lithium carbonate toxicity in a newborn infant. Am J Dis Child 1981; 135:941–943Medline, Google Scholar
37. Filtenborg JA: Persistent pulmonary hypertension after lithium intoxication in the newborn. Eur J Pediatr 1982; 138:321–323Crossref, Medline, Google Scholar
38. Krause S, Ebbesen F, Lange AP: Polyhydramnios with maternal lithium treatment. Obstet Gynecol 1990; 75:504–506Medline, Google Scholar
39. Morrell P, Sutherland GR, Buamah PK, Oo M, Bain HH: Lithium toxicity in a neonate. Arch Dis Child 1983; 58:539–541Crossref, Medline, Google Scholar
40. Rane A, Tomson G, Bjarke B: Effects of maternal lithium therapy in a newborn infant. J Pediatr 1978; 93:296–297Crossref, Medline, Google Scholar
41. Stevens D, Burman D, Midwinter A: Transplacental lithium poisoning (letter). Lancet 1974; 2:595Crossref, Medline, Google Scholar
42. Stothers JK, Wilson DW, Royston N: Lithium toxicity in the newborn. BMJ 1973; 3:233–234Crossref, Medline, Google Scholar
43. Tunnessen WW, Hertz CG: Toxic effects of lithium in newborn infants: a commentary. J Pediatr 1972; 81:804–807Crossref, Medline, Google Scholar
44. Wilbanks GD, Bressler B, Peete CH, Cherny WB, London WL: Toxic effects of lithium carbonate in a mother and newborn infant. JAMA 1970; 213:865–867Crossref, Medline, Google Scholar
45. Wilson N, Forfar JC, Godman MJ: Atrial flutter in the newborn resulting from maternal lithium ingestion. Arch Dis Child 1983; 58:538–539Crossref, Medline, Google Scholar
46. Woody JN, London WL, Wilbanks GD: Lithium toxicity in a newborn. Pediatrics 1971; 47:94–96Medline, Google Scholar
47. Zegers B, Andriessen P: Maternal lithium therapy and neonatal morbidity. Eur J Pediatr 2003; 162:348–349Crossref, Medline, Google Scholar
48. Mizrahi EM, Hobbs JF, Goldsmith DI: Nephrogenic diabetes insipidus in transplacental lithium intoxication. J Pediatr 1979; 94:493–495Crossref, Medline, Google Scholar
49. Frassetto F, Martel FT, Barjhoux CE, Villier C, Le Bot B, Vincent F: Goiter in a newborn exposed to lithium in utero. Ann Pharmacother 2002; 36:1745–1748Crossref, Medline, Google Scholar
50. Karlsson K, Lindstedt G, Lundberg PA, Selstam U: Transplacental lithium poisoning: reversible inhibition of fetal thyroid (letter). Lancet 1975; 1:1295Crossref, Medline, Google Scholar
51. Silverman JA, Winters RW, Strande C: Lithium carbonate therapy during pregnancy: apparent lack of effect upon the fetus. Am J Obstet Gynecol 1971; 109:934–936Crossref, Medline, Google Scholar
52. Flaherty B, Krenzelok EP: Neonatal lithium toxicity as a result of maternal toxicity. Vet Hum Toxicol 1997; 39:92–93Medline, Google Scholar
53. Nishiwaki T, Tanaka K, Sekiya S: Acute lithium intoxication in pregnancy. Int J Gynaecol Obstet 1996; 52:191–192Crossref, Medline, Google Scholar
54. Aoki FY, Ruedy J: Severe lithium intoxication: management without dialysis and report of a possible teratogenic effect of lithium. CMAJ 1971; 105:847–848Medline, Google Scholar
55. Fries H: Lithium in pregnancy. Lancet 1970; 1:1233Crossref, Medline, Google Scholar
56. MacKay AVP, Loose R, Glen AIM: Labour on lithium. BMJ 1976; 1:878Crossref, Medline, Google Scholar
57. Mallinger AG, Hanin I, Stumpf RL, Mallinger J, Kopp U, Erstling C: Lithium treatment during pregnancy: a case study of erythrocyte choline content and lithium transport. J Clin Psychiatry 1983; 44:381–384Medline, Google Scholar
58. Schou M, Amdisen A: Lithium and the placenta (letter). Am J Obstet Gynecol 1975; 122:541Medline, Google Scholar
59. Weinstein MR, Goldfield M: Lithium carbonate treatment during pregnancy: report of a case. Dis Nerv Syst 1969; 30:828–832Medline, Google Scholar
60. Binder ND, Faber JJ, Thornburg KL: The transplacental potential difference as distinguished from the maternal-fetal potential difference of the guinea-pig. J Physiol 1978; 282:561–570Crossref, Medline, Google Scholar
61. Thornburg KL, Binder ND, Faber JJ: Distribution of ionic sulfate, lithium, and bromide across the sheep placenta. Am J Physiol 1979; 236:C58-C65Google Scholar
62. Yonkers KA, Little BB, March D: Lithium during pregnancy: drug effects and their therapeutic implications. CNS Drugs 1998; 9:261–269Crossref, Medline, Google Scholar
63. Yonkers KA, Wisner KL, Stowe Z, Leibenluft E, Cohen L, Miller L, Manber R, Viguera A, Suppes T, Altshuler L: Management of bipolar disorder during pregnancy and the postpartum period. Am J Psychiatry 2004; 161:608–620Link, Google Scholar
64. Krutzen E, Olofsson P, Back SE, Nilsson-Ehle P: Glomerular filtration rate in pregnancy: a study in normal subjects and in patients with hypertension, preeclampsia and diabetes. Scand J Clin Lab Invest 1992; 52:387–293Crossref, Medline, Google Scholar
65. Lafayette RA, Druzin M, Sibley R, Derby G, Malik T, Huie P, Polhemis C, Deen WM, Myers BD: Nature of glomerular dysfunction in preeclampsia. Kidney Int 1998; 54:1240–1249Crossref, Medline, Google Scholar
66. Moran P, Baylis PH, Lindheimer MD, Davison JM: Glomerular ultrafiltration in normal and preeclamptic pregnancy. J Am Soc Nephrol 2003; 14:648–652Crossref, Medline, Google Scholar
67. Sproule B: Lithium in bipolar disorder: can drug concentrations predict therapeutic effect? Clin Pharmacokinet 2002; 41:639–660Crossref, Medline, Google Scholar
68. Varga I, Rigo J, Somos P, Joo JG, Nagy B: Analysis of maternal circulation and renal function in physiologic pregnancies; parallel examinations of the changes in the cardiac output and the glomerular filtration rate. J Matern Fetal Med 2000; 9:97–104Medline, Google Scholar
69. Strevens H, Wide-Swensson D, Torffvit O, Grubb A: Serum cystatin C for assessment of glomerular filtration rate in pregnant and non-pregnant women: indications of altered filtration process in pregnancy. Scand J Clin Lab Invest 2002; 62:141–147Crossref, Medline, Google Scholar



