Prevalence and Natural Course of Aging-Associated Cognitive Decline in a Population-Based Sample of Young-Old Subjects
Abstract
OBJECTIVE: “Mild cognitive impairment” refers to cognitive deficits in older age that exceed age-related cognitive decline but do not fulfill criteria for dementia. Affected subjects are assumed to be at higher risk for the development of dementia, such as Alzheimer’s disease. However, little is known about the group of young-old subjects with respect to the prevalence and natural course of cognitive decline. METHOD: Within the population-based Interdisciplinary Longitudinal Study on Adult Development and Aging, neuropsychological functioning was assessed in 500 community-dwelling young-old subjects of two German urban regions who were born during 1930–1932. The participants were carefully screened for physical and mental health and reexamined 4 years later. The concept of “aging-associated cognitive decline” was applied. RESULTS: At baseline, 13.4% of the subjects fulfilled criteria for aging-associated cognitive decline. Four years later, the prevalence rates for rose to 23.6%; 52.3% of the subjects initially classified as having aging-associated cognitive decline retained the diagnosis at follow-up. Although subjects with aging-associated cognitive decline showed a reduced performance in all neuropsychological domains addressed, a significant decline was confined to delayed verbal memory test performance during the 4-year follow-up period in relation to comparison subjects. Aging-associated cognitive decline did not predict conversion to dementia during the follow-up interval. CONCLUSIONS: In young-old community-dwelling individuals, aging-associated cognitive decline is a frequent condition with a high temporal stability. During a 4-year follow-up, subjects with aging-associated cognitive decline deteriorated specifically in measures of episodic memory, underscoring the value of the respective deficits in characterizing “mild cognitive impairment.”
Mild cognitive impairment refers to cognitive deficits that exceed age-related cognitive decline but do not fulfill criteria for dementia. Clinical and epidemiological evidence have indicated that patients with Alzheimer’s disease undergo a long-standing preclinical phase in which cognitive deficits remain subtle over a long-standing phase before the threshold of dementia is reached (1). Because it has been postulated that elderly subjects with mild cognitive impairment are at an increased risk of developing dementia, a reliable identification of those preclinical stages is important for successful preventive strategies and early therapeutic interventions.
Studies have reported age-dependent prevalence rates of mild cognitive impairment in the elderly population (2, 3). When we take into account that older age is the most important risk factor for Alzheimer’s disease and mild cognitive impairment precedes Alzheimer’s disease in a considerable proportion of affected individuals, it is conceivable that not only the prevalence rates of mild cognitive impairment but also the conversion rates to Alzheimer’s disease differ with respect to selective age ranges within the elderly population (e.g., the young-old, the old-old, and the oldest-old) (4). To date, studies investigating the prevalence and course of mild cognitive impairment have mainly focused on subjects in their 70s and older.
Furthermore, the majority of studies did not address mild cognitive impairment because of significant somatic comorbidity. This is of particular importance since diabetes mellitus, heart disease, and hypertension were recently found to be more prevalent among subjects with mild cognitive impairment than otherwise healthy participants in a longitudinal study (5). This effect could clearly be addressed more thoroughly by the prospective investigation of young-old subjects, in whom these conditions are less frequent.
To date, various research diagnostic criteria as well as clinical manuals have been proposed to further define mild cognitive impairment (2, 6, 7). Those include age-associated memory impairment (8) and its modifications: age-consistent memory impairment (9) and late-life forgetfulness (9), the amnestic variant of mild cognitive impairment introduced by Petersen and colleagues (mild cognitive impairment, amnestic) (10), and the ICD-10 criteria of “mild cognitive disorder.” To meet some of the limitations affecting earlier attempts to define mild cognitive impairment, a working party of the International Psychogeriatric Association introduced the concept of “aging-associated cognitive decline” (11). In contrast to most previous concepts, aging-associated cognitive decline not only uses age- and educational-adjusted normal levels to define a cognitive deficit but also considers decline in a broader potential range of cognitive domains, namely, memory and learning, attention and concentration, thinking, language, and visuospatial functioning. This agrees with the hypothesis that not only mnestic but also language deficits, such as verbal fluency impairment, might indicate further cognitive decline (12).
The use of normal values adjusted for educational levels constitutes another advantage of the aging-associated cognitive decline concept because higher education might be associated with increased cognitive reserve capacity, leading to delayed onset of cognitive decline. Thus, it has been postulated that aging-associated cognitive decline has an improved potential to identify individuals who experience cognitive decline that falls short of dementia (11).
Until now, three longitudinal population-based studies have been performed to establish the prevalence rates of aging-associated cognitive decline in the elderly population and to assess its predictive validity (13–15). Notably, all three studies found similar prevalence rates for aging-associated cognitive decline in the investigated populations (20%–27%). Furthermore, aging-associated cognitive decline proved to be superior to other concepts of mild cognitive impairment with respect to temporal stability and the prediction of dementia, yielding conversion rates of 28%–47% within a 2–3-year period. Although these results indeed support the notion that aging-associated cognitive decline is of high use for the identification of preclinical stages of Alzheimer’s disease, they are not necessarily generalizable to the elderly population as a whole. Because previous studies mainly focused on the group of old-old individuals, little is known about the prevalence and conversion rates of aging-associated cognitive decline for individuals in their 60s. However, the latter age group does not only constitute a major proportion of the individuals asking for advice in outpatient memory clinics but might also represent a promising target population for early preventive interventions. This holds particularly true considering that most of these young-old individuals are still living independently in the community.
Accordingly, the aims of the present study were twofold: 1) to establish prevalence rates for mild cognitive impairment according to criteria for aging-associated cognitive decline within a population-based sample of young-old individuals and 2) to investigate the longitudinal course of this condition, particularly with respect to its temporal stability, neuropsychological test performance, and conversion to dementia.
Method
Subjects and Psychometric Instruments
The subjects were participants in the Interdisciplinary Longitudinal Study on Adult Development and Aging who were born between 1930 and 1932. The Interdisciplinary Longitudinal Study on Adult Development and Aging is a prospective study on adult development in Germany that is based on two birth cohorts born during 1930–1932 and 1950–1952 (16). The subjects were randomly identified and recruited according to community registers. These registers are regularly updated for changes of address and marital status. Because it is compulsory for each resident in Germany ages 16 and older to be registered, this recruitment procedure yielded an almost representative sample for the respective communities.
All 500 participants of the elderly birth cohort who were living in the urban regions of Leipzig (Saxony) and Heidelberg/Mannheim (Palatine) were included in the present study. The study was approved by the ethical committee of the University of Heidelberg. After complete description of the study to the subjects, written informed consent was obtained.
The participants were carefully screened for physical and mental health by extensive interviews, physical examinations, and laboratory tests. In addition, potential psychiatric disorders were assessed by using the German version of the Structured Clinical Interview for the DSM-III-R (17).
Most of the neuropsychological instruments applied for the assessment of cognitive performance were subtests of the Nürnberger-Alters-Inventar(18) and the Leistungsprüfsystem(19), both of which are well established and commonly used test batteries in Germany. In particular, the following instruments were used for the investigation of the respective cognitive domains:
| 1. | Memory and learning—immediate word list recall and delayed word list recognition (Nürnberger-Alters-Inventar) | ||||
| 2. | Attention and concentration—Aufmerksamkeits-Belastungs-Test(20) | ||||
| 3. | Abstract thinking—similarities subtest (Hamburg-Wechsler-Intelligenztest für Erwachsene) (21) | ||||
| 4. | Language—subtest of verbal fluency (Leistungsprüfsystem) | ||||
| 5. | Visuospatial functioning—subtest of visual imagination (Räumliche VorstellungLeistungsprüfsystem) | ||||
Subjective cognitive complaints were assessed by interviewing and applying the appropriate items of the Nürnberger Selbsteinschätzungsliste(22). The Nürnberger Selbsteinschätzungsliste is a self-assessment questionnaire on general functioning in the elderly and contains four items with a direct relation to cognitive functioning. Subjective complaints were explored on a “yes or no” basis. In addition, the Self-Rating Depression Scale (23) was applied.
To date, the first two waves of the Interdisciplinary Longitudinal Study on Adult Development and Aging are complete and served as the database for the present study. Examinations took place between December 1993 and January 1995 (time 1) and between December 1997 and January 2000 (time 2). The mean age of the subjects was 62.4 years (SD=2.4) at baseline (time 1) and 66.7 years (SD=1.1) at the 4-year follow-up (time 2). The sample had a balanced gender distribution (249 women and 251 men).
Definition of Diagnostic Categories
Aging-associated cognitive decline was diagnosed according to the criteria of the International Psychogeriatric Association working party (11). Those include 1) subjective impairment: a report by the individual (or a reliable informant) that cognitive function has declined and 2) objective impairment: difficulties in any of the following cognitive domains, as indicated by a neuropsychological test performance of at least one standard deviation below normal age and educational levels: memory and learning, attention and concentration, abstract thinking (problem solving, abstraction), language, and visuospatial functioning. Age-adjusted normal values were available for all psychometric instruments administered, but normal values adjusted for educational level were missing for the tests addressing verbal fluency and visuospatial functioning. In these instances, the results of the entire age cohort were differentiated according to high (secondary school) and low (primary school) educational levels. In the latter instances, the test results of the entire age cohort (1930–1932) were differentiated according to high (secondary school) and low (primary school) educational levels, and each of the resulting distributions was used as a reference. The same differentiation was applied for the remaining tests on the basis of the normal levels reported in the literature.
Exclusion criteria were that none of the abnormalities listed was of a sufficient degree for a diagnosis of dementia or could be attributed to a clinically significant psychiatric disorder (in particular, depression, substance abuse, or psychosis). Furthermore, there should have been no objective evidence from physical and neurological examinations or laboratory tests and no history of cerebral disease, damage, or dysfunction or of a systemic physical disorder that is known to cause cognitive dysfunction.
As pointed out in the International Psychogeriatric Association consensus paper (11), the differential diagnosis between aging-associated cognitive decline, dementia, and ICD-10 “mild cognitive disorder” should be considered the most important. In our investigation, dementia was defined in line with DSM-IV criteria. In short, this includes the development of multiple cognitive deficits that are severe enough to cause significant impairment in social or occupational functioning. The diagnosis of mild cognitive disorder was assigned if a mild cognitive deficit according to the two criteria for aging-associated cognitive decline was present, but a history and/or an objective examination revealed evidence for a cerebral and/or systemic disorder that was sufficient to cause cerebral dysfunction (exclusion criterion for aging-associated cognitive decline).
Data Analysis
Complete data sets were available for 485 of the 500 investigated subjects. For each time point of the investigation, prevalence rates for aging-associated cognitive decline, mild cognitive disorder, and dementia were determined according to the criteria just described. Furthermore, conversion rates from one to another diagnostic category were calculated. Repeated-measures analysis of variance (ANOVA) was used to analyze the time course of cognitive deficits across diagnostic groups with respect to different cognitive domains.
Results
At time 1, subjective complaints about cognitive decline were found in 226 subjects (46.6%), and 200 subjects (41.2%) scored below one standard deviation of age- and education-adjusted normal values on at least one of the cognitive tests applied. Ninety-two subjects (19.0%) of the total sample suffered from medical and/or neuropsychiatric conditions with a potential causative relation to cognitive decline. When diagnostic criteria were applied to these findings, 65 subjects (13.4%) fulfilled the criteria for aging-associated cognitive decline. Additionally, 28 subjects (5.8%) had evidence of subjective and objective cognitive impairment but simultaneously met the exclusion criteria of aging-associated cognitive decline. Those were classified as suffering from mild cognitive disorder. None of the investigated participants was diagnosed as suffering from dementia according to the DSM-IV criteria; data sets from 15 subjects had to be excluded owing to missing values.
At time 2, after a follow-up period of 4 years, 449 subjects, or 89.8% of the original sample, could be reexamined. Twenty subjects had died. Other reasons for dropout (N=31) were severe physical handicaps that would make the investigation too troublesome, lost of interest/motivation, having moved to other places in the country, or no reason given for refusal. Dropout rates were highest in the subjects with aging-associated cognitive decline, followed by those fulfilling ICD-10 criteria for mild cognitive disorder, and then comparison subjects (15.4%, 14.3%, and 7.6%, respectively); however, these differences did not reach statistical significance (χ2=3.2, df=2, n.s.). Among the subjects who were reexamined at time 2, the prevalence rate of aging-associated cognitive decline increased to 23.6% (N=106). An additional 7.8% of the subjects (N=35) fulfilled the ICD-10 criteria for mild cognitive disorder. None of the reexamined subjects had developed dementia during the 4-year follow-up.
Of the subjects diagnosed with aging-associated cognitive decline at time 1, 34 (52.3%) retained the diagnosis at follow-up (Figure 1). Two subjects (3.1%) no longer complained about cognitive decline, and in another three subjects (4.6%), a severe medical condition sufficient to cause cognitive dysfunction had become apparent during the follow-up period. Both conditions led to an exclusion from the group with aging-associated cognitive decline. In contrast, 15 subjects (23.1%) of the original group with aging-associated cognitive decline performed within normal limits (one standard deviation) on cognitive testing at the follow-up, despite persisting complaints about cognitive decline. Another subject classified as having aging-associated cognitive decline at time 1 had no subjective or objective cognitive deficits (1.5%). Ten subjects (15.4%) who were initially diagnosed with aging-associated cognitive decline dropped out and could not be reexamined at time 2. Of the 207 subjects with subjective complaints at baseline, 172 reproduced their complaints at follow-up.
Although four of the 28 subjects who initially fulfilled criteria for mild cognitive disorder could not be reexamined, seven of them no longer demonstrated cognitive impairment at follow-up. Among those, two subjects no longer reported subjective complaints, whereas five performed at normal levels in all tests applied.
At time 2, 72 new cases of aging-associated cognitive decline were identified (Figure 2). Among those, 17 had been found to be unimpaired on cognitive testing at time 1, although 20 of them had already complained about cognitive decline at the time of the first examination. Another 35 incidence patients with aging-associated cognitive decline had previously been impaired on cognitive testing but did not fulfill the subjective criteria at time 1.
At time 1, the majority of subjects with aging-associated cognitive decline demonstrated cognitive deficits on one or two (N=28, 82.3%) neuropsychological tests, whereas deficits in three or four (N=6, 17.7%) domains were less frequent. At time 2, the proportion of subjects with aging-associated cognitive decline who were impaired on one or two (N=22, 64.7%) tests had lower ratings, whereas more subjects showed deficits in three or more domains (N=12, 35.3%) (χ2=2.7, df=1, p=0.09).
The prevalence of isolated mnestic deficits according to “mild cognitive impairment, amnestic” (10) was rather low. The respective criteria were applied for 4.1% of the subjects at time 1 and 4.2% at time 2, respectively. A total of 27.8% (N=5) of the subjects who fulfilled the criteria for “mild cognitive impairment, amnestic” at time 1 could be reclassified accordingly at time 2.
In a further step, the development of performance across different cognitive domains during the follow-up period was compared between subjects who retained the diagnosis of aging-associated cognitive decline from time 1 to time 2 (N=34) and comparison subjects who were cognitively unimpaired both at time 1 and time 2 (N=39) (Table 1). According to the ANOVAs calculated, the subjects with aging-associated cognitive decline were significantly impaired on all cognitive tests applied in relation to the comparison subjects (word list immediate recall—diagnosis main effect: F=39.2, df=1, 71, p<0.0005; time main effect: F=0.3 df=1, 71, p=0.60; word list delayed recognition—diagnosis main effect: F=16.8, df=1, 71, p<0.0005; time main effect: F=0.5, df=1, 71, p=0.50; Aufmerksamkeits-Belastungs-Test [attention and concentration]—diagnosis main effect: F=16.1, df=1, 69, p<0.0005; time main effect: F=0.2, df=1, 69, p=0.60; Hamburg-Wechsler-Intelligenztest für Erwachsene similarities subtest [abstract thinking]—diagnosis main effect: F=24.8, df=1, 71, p<0.0005; time main effect: F=4.6, df=1, 71, p<0.05; verbal fluency—diagnosis main effect: F=36.8, df=1, 71, p<0.0005; time main effect: F=3.5, df=1, 71, p=0.06; and visual imagination—diagnosis main effect: F=19.8, df=1, 71, p<0.0005; time main effect: F=14.2, df=1, 71, p<0.0005).
Additionally, the subjects with aging-associated cognitive decline showed a significant decline in performance on the delayed word recognition task in relation to the comparison subjects during the follow-up, as demonstrated by a significant diagnosis-by-time interaction (word list delayed recognition—diagnosis-by-time interaction: F=4.3, df=1, 71, p<0.05). The respective interaction for verbal fluency also reached the significance level (verbal fluency—diagnosis-by-time interaction: F=4.4, df=1, 71, p<0.05). However, this effect resulted from an increased performance in the comparison subjects, whereas the subjects with aging-associated cognitive decline showed rather stable values. None of the other interactions for the remaining neuropsychological test scores addressing immediate recall, attention and concentration, abstract thinking, and visuospatial functioning reached significance (0.1<F<2.9, df=1, 71).
In relation to the comparison subjects, the subjects with aging-associated cognitive decline demonstrated significantly (diagnosis main effect: F=24.4, df=1, 71, p<0.005) more mild depressive symptoms at time 1 (Self-Rating Depression Scale score—subjects with aging-associated cognitive decline: mean=37.7, SD=7.8; comparison subjects: mean=30.3, SD=5.6) and time 2 (Self-Rating Depression Scale score—subjects with aging-associated cognitive decline: mean=38.0, SD=7.5; comparison subjects: mean=30.4, SD=5.7). Similar results were obtained when all subjects diagnosed with aging-associated cognitive decline at time 2 (N=106) were compared with all cognitively unimpaired comparison subjects at time 2 (N=245).
Discussion
According to our findings, aging-associated cognitive decline represents a frequent condition affecting 13.4% of the 60–64-year-old population. In addition, the prevalence of aging-associated cognitive decline showed an age-related increase that rose to 23.6% within a 4-year follow-up period. The established prevalence rates are in line with the results from previous population-based studies that applied the criteria for aging-associated cognitive decline (13–15). However, although previous investigations mainly focused on subjects in their 70s and 80s, our study for the first time, to our knowledge, indicates that aging-associated cognitive decline is also common in the population of young-old. Because all subjects underwent a thorough physical examination, the prevalence rates of aging-associated cognitive decline in the present study may not be attributed to the early effects of severe systemic or neurological disorders.
Furthermore, the diagnosis of aging-associated cognitive decline was characterized by a relatively high temporal stability. This finding emphasizes the existence of a distinct diagnostic entity, such as mild cognitive impairment, that has been challenged (24, 25). It has been argued that mild cognitive impairments might considerably fluctuate over time and would not be suitable to define a circumscribed diagnostic category. Indeed, several population-based studies revealed that, in particular, the amnestic form of mild cognitive impairment (mild cognitive impairment, amnestic) (10) was rather unstable over time, in that only about 7% of the affected subjects were still classified under this category after 2 to 3 years, whereas more than 40% reverted to normal (14, 25). This is in contrast to our finding that 52.3% of the subjects with a baseline diagnosis of aging-associated cognitive decline retained the diagnosis at follow-up. Furthermore, 6.2% had persisting deficits in cognitive tests, although they had to be excluded from the group with aging-associated cognitive decline for other reasons (lack of subjective complaints, severe medical conditions). Only 23.1% of the original subjects with aging-associated cognitive decline improved with respect to cognitive testing. A single person (1.5%) reverted to normal, insofar as he was neither subjectively nor objectively impaired at follow-up. Similar data were reported by Ritchie et al. (14), who found that 50%–60% of the subjects classified as having aging-associated cognitive decline retained this diagnosis when they were reexamined after 1 year. Taken together, these findings indicate that in contrast to other concepts of mild cognitive impairment, aging-associated cognitive decline defines a distinct syndrome that is reproducible over time in a considerable proportion of elderly subjects.
In relation to the comparison subjects, a significantly larger percentage of subjects with aging-associated cognitive decline suffered from mild depressive symptoms. Because patients with manifest depression were excluded from the group with aging-associated cognitive decline, cognitive impairment due to affective disorders did not contribute to this finding. Otherwise, in patients with manifest dementia, depressive symptoms and apathy often overlap, making it difficult to differentiate depressive disorder comorbidity in dementia. Thus, mild depressive symptoms in aging-associated cognitive decline might represent an epiphenomenon of increasing cognitive decline.
In our population of the young-old, aging-associated cognitive decline did not predict conversion to dementia within a 4-year follow-up period. Ritchie et al. (14) and Busse et al. (15) determined conversion rates of 28% and 47% within a comparable time interval, respectively. However, the investigated population in these studies was considerably older. Dropout rates were highest in subjects with aging-associated cognitive decline, followed by those fulfilling ICD-10 criteria for mild cognitive disorder, and then comparison subjects, and it is likely that at least some of the subjects with aging-associated cognitive decline might have converted into dementia. However, we could not definitely prove this assumption. Nevertheless, other factors must be considered to explain this discrepancy. In particular, length of the follow-up interval has to be weighed against the age of the subjects. Previous studies on cognitive deficits in preclinical Alzheimer’s disease have revealed some empirical evidence that deficits across multiple cognitive domains are apparent not only years but even decades before the diagnosis of dementia can be made (26) and that the magnitude of those preclinical cognitive deficits appears to be relatively stable until a few years before the clinical diagnosis is made (1). Consequently, the likelihood of observing accelerated changes in cognitive performance among incident Alzheimer’s disease increases as time before the eventual diagnosis decreases. When we take into account that the incidence and prevalence of dementia are relatively low during the seventh decade of life but increase exponentially from the age of 70, the divergent results with respect to conversion rates might mainly be explained by age differences between the studied populations. Accordingly, our results suggest that age and length of follow-up interval are of crucial relevance when the predictive validity of different concepts of mild cognitive impairment is assessed.
Nonetheless, longitudinal analysis of our data indicates that the respective neuropsychological deficits follow a progressive course. At time 2, the subjects with aging-associated cognitive decline were impaired on more neuropsychological tests than at time 1. This observation is in line with the assumption that the putative pathological process involved a broader range of cognitive domains during the follow-up period. In relation to comparison subjects, the subjects with aging-associated cognitive decline deteriorated significantly during follow-up in the test on delayed word recognition. In contrast, performance on immediate recall remained rather stable in the subjects with aging-associated cognitive decline or improved slightly in the comparison subjects. Obviously, the slight performance gains observed in the comparison group in this cognitive domain, as well as with respect to delayed recognition and verbal fluency, may refer to an increased awareness of the participants of the study toward their neuropsychological performance. Moreover, the double dissociation found between immediate and delayed verbal memory argues against an effect of other conditions, such as depressive syndromes, that should affect both recall conditions simultaneously.
Several previous studies have addressed the question of whether deficits in specific cognitive domains predict the development of Alzheimer’s disease (26–31). They all agreed that, in particular, deficits with respect to episodic memory tasks but also, to a lesser degree, other cognitive dysfunctions are associated with an increased risk of further cognitive decline. Notably, Palmer et al. (30) also found that specific tests of word recall and verbal fluency had positive predictive value for dementia. These findings are paralleled by the results of recent neuroimaging studies. Pantel et al. (32) demonstrated (in a subsample of the subjects with aging-associated cognitive decline investigated here) atrophic changes of medial temporal lobe structures that are specifically involved in episodic memory function. Similar findings were obtained in a variety of previous neuroimaging studies in patients with manifest Alzheimer’s disease (for a review, see references 33 and 34).
In conclusion, we show that aging-associated cognitive decline is a frequent condition in community-dwelling young-old subjects. The prevalence of aging-associated cognitive decline increased with age, and the diagnosis of aging-associated cognitive decline was characterized by a rather high temporal stability. However, in this particular sample of young-old subjects, aging-associated cognitive decline did not predict dementia during a 4-year follow-up period. The subjects with aging-associated cognitive decline were characterized by a selective further decline of episodic memory that occurred independently from physical comorbidity.
 |
Received Aug. 2, 2004; revision received Nov. 9, 2004; accepted Dec. 3, 2004. From the Section for Geriatric Psychiatry and the Institute of Gerontology, University of Heidelberg; and the Department of Psychiatry and Psychotherapy, University of Frankfurt am Main, Frankfurt, Germany. Address correspondence and reprint requests to Dr. Johannes Schröder, Section for Geriatric Psychiatry, Ruprecht-Karls University Heidelberg, Voss-Str. 4, D-69115 Heidelberg, Germany; [email protected] (e-mail). Supported by the Forschungsprogramm des Landes Baden-Württemberg and the Federal Ministry for Family, Senior Citizen, Women, and Youth. The authors thank Dr. J. Geider for providing statistical expertise.
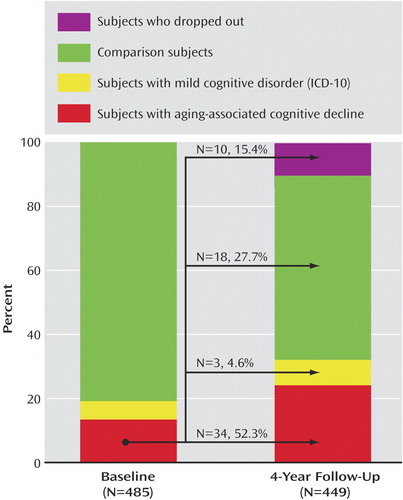
Figure 1. Diagnostic Classification of Young-Old Subjects With Aging-Associated Cognitive Decline or Mild Cognitive Disorder and Comparison Subjects at Baseline (Time 1) and 4-Year Follow-Up (Time 2)a
aThe arrows signify the status of 65 subjects with aging-associated cognitive decline at time 1 by time 2.
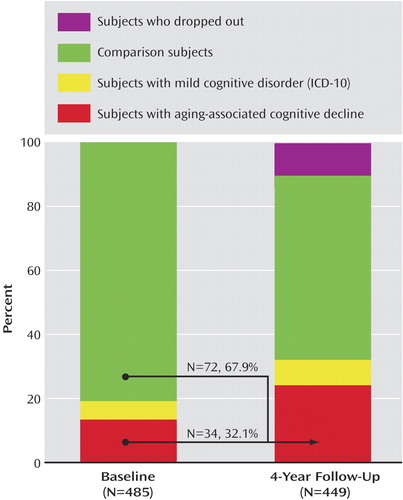
Figure 2. Initial Diagnostic Classification of Young-Old Subjects With Aging-Associated Cognitive Decline or Mild Cognitive Disorder and Comparison Subjects at Baseline (Time 1) and 4-Year Follow-Up (Time 2)a
aThe arrows signify the status of 106 subjects with aging-associated cognitive decline at time 2 by time 1.
1. Small BJ, Mobly JL, Laukka EJ, Jones S, Backman L: Cognitive deficits in preclinical Alzheimer’s disease. Acta Neurol Scand Suppl 2003; 179:29–33Crossref, Medline, Google Scholar
2. Bischkopf J, Busse A, Angermeyer MC: Mild cognitive impairment: a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand 2002; 106:403–414Crossref, Medline, Google Scholar
3. Tervo S, Kivipelto M, Hänninen T, Vanhanen M, Hellikainen M, Mannermaa A, Soininen H: Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord 2004; 17:196–203Crossref, Medline, Google Scholar
4. Riley MW, Riley JW: Longevity and social structure: the potential of the added years, in Our Aging Society: Paradox and Promise. Edited by Pifer A, Bronte L. New York, WW Norton, 1986, pp 53–77Google Scholar
5. Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH: Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study, part 2. Arch Neurol 2003; 60:1394–1399Crossref, Medline, Google Scholar
6. Schröder J, Kratz B, Pantel J, Minnemann E, Lehr U, Sauer H: Prevalence of mild cognitive impairment in an elderly community sample. J Neural Transm 1998; 54:51–59Crossref, Google Scholar
7. Ritchie K, Touchon J: Mild cognitive impairment: conceptual basis and current nosological status. Lancet 2000; 335:225–228Crossref, Google Scholar
8. Crook T, Bartus RT, Ferris SH, Whitehouse P, Cohen GD, Gershon S: Age associated memory impairment: proposed diagnostic criteria and measures of clinical change: report of a National Institute of Mental Health Work Group. Dev Neuropsychol 1986; 2:261–276Crossref, Google Scholar
9. Blackford RC, La Rue A: Criteria for diagnosing age-associated memory impairment: proposed improvements from the field. Dev Neuropsychol 1989; 5:295–306Crossref, Google Scholar
10. Petersen CP, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B: Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992Crossref, Medline, Google Scholar
11. Levy R: Aging-associated cognitive decline. Int Psychogeriatr 1994; 6:63–68Crossref, Medline, Google Scholar
12. Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Thal LJ, Saitoh T: Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology 1995; 45:2203–2206Crossref, Medline, Google Scholar
13. Hänninen T, Koivisto K, Reinikainen KJ, Helkala EL, Soininen H, Mykkänen L, Laakso M, Riekkinen PJ: Prevalence of ageing-associated cognitive decline in an elderly population. Age Ageing 1996; 25:201–205Crossref, Medline, Google Scholar
14. Ritchie K, Arteron S, Touchon J: Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001; 56:37–42Crossref, Medline, Google Scholar
15. Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC: Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria. Br J Psychiatry 2003; 182:449–454Crossref, Medline, Google Scholar
16. Martin P, Martin M: Design und Methodik der Interdisziplinären Längsschnittstudie des Erwachsenenalters, in Aspekte der Entwicklung im mittleren und höheren Erwachsenenalter: Ergebnisse der Interdisziplinären Längsstudie des Erwachsenenalters (ILSE). Edited by Martin P, Etrich KU, Lehr U, Roether D, Fischer-Cyrulies A. Darmstadt, Germany, Steinkopf, 2000, pp 17–27Google Scholar
17. Wittchen HU, Zaudig M, Schramm E, Spengler P, Mombour W, Klug J, Horn R: Strukturiertes klinisches Interview für DSM-III-R. Göttingen, Germany, Beltz-Test, 1991Google Scholar
18. Oswald WD, Fleischmann VM: Nürnberger-Alters-Inventar. Erlangen-Nürnberg, Germany, Universität Erlangen-Nürnberg, 1991Google Scholar
19. Horn WC: Leistungsprüfsystem. Göttingen, Germany, Hogrefe, 1983Google Scholar
20. Brickenkamp R: Test d2: Aufmerksamkeits-Belastungs-Test. Göttingen, Germany, Hogrefe, 1978Google Scholar
21. Tewes W: HAWIE-R: Hamburg-Wechsler-Intelligenztest für Erwachsene, Revision. Bern, Germany, Huber, 1991Google Scholar
22. Oswald WD, Fleischmann VM: Nürnberger Selbsteinschätzungsliste (NSL). Erlangen-Nürnberg, Germany, Universität Erlangen-Nürnberg, 1986Google Scholar
23. Zung WW, Richards CB, Short MJ: Self-Rating Depression Scale in an outpatient clinic: further validation of the SDS. Arch Gen Psychiatry 1965; 13:508–515Crossref, Medline, Google Scholar
24. Milwain E: Mild cognitive impairment: further caution (letter). Lancet 2000; 355:1018Crossref, Medline, Google Scholar
25. Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, Barberger-Gateau P, Dartigues JF: Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599Crossref, Medline, Google Scholar
26. Tierney MC, Szalai JP, Snow WG, Fisher RH, Nores A, Nadon G, Dunn E, St George-Hyslop PH: Prediction of probable Alzheimer’s disease in memory-impaired patients: a prospective longitudinal study. Neurology 1996; 46:661–665Crossref, Medline, Google Scholar
27. Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB: The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham cohort. Arch Neurol 2000; 57:808–813Crossref, Medline, Google Scholar
28. Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M: Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry 2001; 58:853–858Crossref, Medline, Google Scholar
29. Tian J, Bucks RS, Haworth J, Wilcock G: Neuropsychological prediction of conversion to dementia from questionable dementia: statistically significant but not yet clinically useful. J Neurol Neurosurg Psychiatry 2003; 74:433–438Crossref, Medline, Google Scholar
30. Palmer K, Backman L, Winblad B, Fratiglioni L: Detection of Alzheimer’s disease and dementia in the preclinical phase: population based cohort study. BMJ 2003; 326:245Crossref, Medline, Google Scholar
31. Backman L, Small BJ, Fratiglioni L: Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 2001; 124:96–102Crossref, Medline, Google Scholar
32. Pantel J, Kratz B, Essig M, Schröder J: Parahippocampal volume deficits in subjects with aging-associated cognitive decline. Am J Psychiatry 2003; 160:379–382Link, Google Scholar
33. Pantel J, Schönknecht P, Essig M, Schröder J: Distribution of cerebral atrophy assessed by magnetic resonance imaging reflects patterns of neuropsychological deficits in Alzheimer’s dementia. Neurosci Lett 2004; 361:17–20Crossref, Medline, Google Scholar
34. Schröder J, Buchsbaum MS, Shihabuddin L, Tang C, Wei T, Spiegel-Cohen J, Hazlett EA, Abel L, Luu-Hsia C, Ciaravolo TM, Marin D, Davis KL: Patterns of cortical activity and memory performance in Alzheimer’s disease. Biol Psychiatry 2001; 49:426–436Crossref, Medline, Google Scholar



