Persistent Choreoathetosis in a Fatal Olanzapine Overdose: Drug Kinetics, Neuroimaging, and Neuropathology
Atypical antipsychotics are a relatively new group of medications for the management of psychosis. Currently approved medications of this category include clozapine, risperidone, quetiapine, and olanzapine. Olanzapine was first marketed in 1996 in the United States as an atypical antipsychotic medication similar to clozapine for schizophrenia. By 2002, olanzapine was widely used and in the top 20 drugs by sales (1). Olanzapine belongs to the thienobenzodiazepine drug class and is known to have a high affinity for dopamine D2, D3, and D4 receptors, all five serotonin HT2 receptor subtypes, the 5-HT6 receptor, acetylcholine muscarinic receptors, and α1-adrenergic and histamine H1 receptors (2). The drug is well absorbed from the gut and reaches maximum plasma concentration about 6 hours after an oral dose (3). In plasma, 93% of the drug is bound to serum proteins, especially albumin (3). The drug is extensively eliminated by first-pass metabolism in the liver and has a mean elimination half-life of 30 hours, with a range of 21–54 hours (3).
Olanzapine is considered to have a good overall safety profile at therapeutic doses (4). Adverse effects of olanzapine that affect the nervous system include mental status deterioration and extrapyramidal signs and, rarely, delirium, mutism, confusion, aggression, and lethargy that can progress to coma. Seizures, status epilepticus, and hypersalivation occasionally develop (5, 6). Extrapyramidal signs may include dystonia, parkinsonism, akathisia, choreoathetosis, and neuroleptic malignant syndrome (7–9). Serious systemic complications include diabetes mellitus, tachycardia and supraventricular tachycardia, arrhythmias, and cardiopulmonary arrest (10, 11).
Overdoses can lead to deaths. The American Association of Poison Control Centers Toxic Exposure Surveillance System reported the number of deaths associated with olanzapine to be six in 2000 (12), 10 in 2001 (13), and 10 in 2002 (14). Before some of these deaths, concomitant agents were ingested. The majority of deaths occurred within 12 hours of overdose, and the results of autopsies, when available, were often unremarkable (15–17). We report here a patient who survived the early phase of an overdose. He then developed coma with persistent choreoathetosis and hypersalivation, and neuroimaging and autopsy findings suggested damage to the basal ganglia.
Case Presentation
History
A 62-year-old married man who had a long and clear history of bipolar affective disorder with features of obsessive compulsion, Mr. A was treated by psychiatrists for more than 10 years. In his 20s he made two suicide gestures with sedatives and cut his wrist. Two years before the current admission he was hospitalized for manic behavior with psychotic features, paranoid delusions, and suicide ideation, but he had made no suicide attempts in the past 30 years. He had no history of cardiac, renal, liver, or pulmonary disease. His last physical examination, 2 months earlier, produced normal results. He regularly took olanzapine (30 mg at bedtime) and lithium (600 mg in 10 ml of syrup twice a day). Twenty years earlier he had intermittently abused alcohol and used marijuana. He was unemployed and lived with his wife, who was not aware of any recent depression or unusual behavior. They had recently taken a vacation, which he was thought to have enjoyed.
Mr. A’s wife returned home from work one evening to find her husband stumbling around the house with slurred, unintelligible speech. He responded appropriately to simple verbal commands. His wife discovered the new 60-pill olanzapine bottle empty and lying on the bedroom floor. She estimated that Mr. A had taken 50 tablets, each of 15 mg (750 mg total). The lithium bottle was still full, and the containers of his other medicines (ibuprofen, terazosin, rabeprazole, methocarbamol, and thiamine) were found not to be opened or missing pills. By ambulance he was taken to the emergency room.
On admission Mr. A had a temperature of 97°F, blood pressure of 134/81 mm Hg, a pulse of 125 bpm, a respiratory rate of 18 breaths/minute, and finger pulse oxygen saturation of 93%. He was confused, restless, and lethargic but was easily aroused and followed some commands. The results of a general physical examination were unremarkable. A neurologic examination demonstrated intact cranial nerves, normal symmetrical motor strength, hyperactive reflexes, and limb ataxia. Table 1 lists the laboratory findings. Blood ethanol measurement and a urine toxicology screen for opioids, amphetamines, benzodiazepines, barbiturates, cocaine, and cannabis were negative. Cerebrospinal fluid from a lumbar puncture was normal. Cranial computed tomography (CT) showed only mild age-related volume loss. The admitting diagnosis was olanzapine overdose with delirium. Mr. A was given oral charcoal and intravenous saline.
In the intensive care unit he became more lethargic and developed copious oral secretions requiring frequent mouth suctioning. He then became semicomatose and was given nasal oxygen with continuous cardiac monitoring because olanzapine overdose is associated with cardiac arrhythmias. Several hours later he developed a brief run of ventricular tachycardia followed by cardiac asystole that lasted less than 2 minutes before returning to sinus rhythm. The cardiac arrhythmia was rapidly detected, and immediate resuscitation was given. Mr. A’s oxygen saturation never fell below 90% when measured by finger pulse oximetry or by three arterial blood gas measurements that day. Transient elevations of his serum lactic acid level to 4.0 mmol/liter (normal, 0.7–2.1) and his creatinine kinase level to 5222 U/liter (normal, <720) occurred. He was intubated for airway protection from his copious secretions, but he breathed spontaneously and never required mechanical ventilation. Brief runs of atrial fibrillation were noted on the cardiac monitor for several days.
On day 3 Mr. A remained semicomatose. His temperature was normal and remained normal during the hospitalization. He developed periods of coarse horizontal nystagmus beating to the right that increased over hours until they were continuous. No limb movements suggestive of a generalized seizure were seen. A second cranial CT on day 3 was initially interpreted as normal. Upon later reexamination, the CT showed subtle low attenuation in both putamen that was not present on the admission CT but corresponded to abnormalities seen on the later magnetic resonance imaging (MRI) scan. An EEG demonstrated frequent small amplitude spike and wave complexes occurring everywhere throughout the tracing but with a predominance in both the frontocentral temporal and anterior temporal regions. A diagnosis of nonconvulsive status epilepticus was made, and Mr. A was given intravenous fosphenytoin (15 mg/kg). The nystagmus stopped but then intermittently returned with hippus requiring periodic administration of intravenous lorazepam (2 mg). A loading dose of intravenous valproate (3000 mg over 6 hours) was then given. The periods of nystagmus slowly subsided over several days. No seizure activity with limb movements ever was noted. A repeat EEG 10 days later showed background activity in the range of 4–6 and 6–8 Hz, poor organization, and infrequent bursts of sharp slow complexes that remained predominately frontal. No burst-suppression electrical activity was seen on any EEG. The results of a transthoracic echocardiogram were normal.
Over the next 2 weeks, Mr. A’s level of consciousness improved slightly; he would open his eyes and turn his head toward voices but would not track people. His vital signs remained normal except for a few periods of paroxysmal atrial fibrillation, which were treated with metoprolol. He remained intubated because of copious salivary secretions but breathed spontaneously. He had normal cranial nerve reflexes, vigorous spontaneous limb movements, and hyperreflexia with bilateral Babinski signs. Occasional myoclonic jerks independently appeared in all limbs.
From week 3 until his death, Mr. A was comatose but had vigorous and frequent choreoathetosis coupled with elements of dystonia that involved his head and all limbs. He banged his limbs and head against the bed rails and developed bruises. At times his arms briefly assumed a decerebrate posture. Over the weeks a variety of medications (lorazepam, risperidone, haloperidol, benztropine, and morphine) were tried to lessen the vigor of the continuous choreoathetosis, with minimal benefit. MRI at week 6 demonstrated mild age-related cerebral atrophy and bilateral small hyperintense foci in the medial putaminis and globus pallida and left caudate head, mainly on diffusion-weighted images and slightly on T2-weighted images but not on T1-weighted images (Figure 1). Mr. A’s EEG slightly improved, with only rare spikes against a slow background activity of 4–7 Hz. A tracheostomy was performed, as he continued to produce copious oral secretions, and a gastrostomy was done for feeding. Late complications included pneumonia, sepsis, and skin infections of the bruised areas on Mr. A’s head and limbs. He remained comatose with choreoathetosis until his death on day 57 from congestive heart failure and pneumonia. An autopsy was performed 16 hours after his death.
Olanzapine Drug Kinetics
Olanzapine and olanzapine metabolites were measured since there is limited information on the human pharmacokinetics of olanzapine overdose. The plasma or sera samples were immediately frozen at –20°C after separation from red blood cells by centrifugation or after clotting. Sera, plasma, and CSF were sent frozen on dry ice to Bioanalytical Systems, West Lafayette, Ind., where the olanzapine assays were performed. A high-performance liquid chromatography method developed by the olanzapine manufacturer was used to determine the concentration of olanzapine in serum, plasma, and CSF (18). The lower and upper limits of quantitation were 0.25 and 100 ng/ml with an interday coefficient of variation of 6.49% at 0.25 ng/ml and 1.71% at 100 ng/ml. Major metabolites of olanzapine (olanzapine-10-N-glucuronide and N-desmethyl olanzapine) were measured in order to determine whether Mr. A’s drug metabolism had been unusual. The metabolite levels were measured by a method similar to that used to measure olanzapine levels. The olanzapine-10-N-glucuronide level for a sample is determined by the difference in olanzapine levels before and after acid hydrolysis. Olanzapine and N-desmethyl olanzapine levels in human plasma have been shown to be stable at –20°C for at least 90 days. The stability of olanzapine-10-N-glucuronide levels has not been tested at Bioanalytical Systems. The elimination half-life for olanzapine in blood was determined by using PK Solutions 2.0 (Summit Research Services, Montrose, Colo.).
Table 2 lists key olanzapine, glucuronide olanzapine, and N-desmethyl olanzapine levels in serum and plasma by time after hospitalization, as the exact time of ingestion that day was unknown. The peak serum olanzapine level was 464.3 ng/ml, which was considerably elevated. Mr. A’s elimination half-life for olanzapine was 52.5 hours, which is similar to the 21–54 hours reported by the manufacturer for elimination of the drug at therapeutic levels (Figure 2) (3). The highest glucuronide olanzapine level was 900.2 ng/ml, and the highest N-desmethyl olanzapine level was 40.8 ng/ml.
Postmortem Findings
The autopsy demonstrated gross and microscopic evidence of bilateral bronchopneumonia. The other internal organs were unremarkable. The brain grossly demonstrated mild cortical atrophy and enlargement of the lateral ventricles without grossly visible lesions in the cerebral cortex, white matter, basal ganglia, brainstem, or cerebellum (Figure 3). After fixation in 10% formalin for 2 weeks, microscopic sections of the striatum, globus pallidus, and thalamus bilaterally showed extensive reactive astrogliosis with intact neuronal populations. Glial fibrillary acidic protein staining highlighted numerous stellate reactive astrocytes, confirming reactive astrocytosis (Figure 3 inset). In addition, there was patchy eosinophilic degeneration on neurons, indicative of acute terminal ischemia and nonspecific loss of the Purkinje cell layer in the cerebellum. Other brain regions were unaffected.
Discussion
The history, pill counts, and laboratory findings indicate that Mr. A overdosed only with olanzapine in an apparent suicide attempt. Although he was taking other medications, those pill bottles were not found open, his blood level of lithium was normal, and the result of a urine toxicology screen at the time of the emergency room examination was negative. He was hospitalized while conscious but lethargic and confused. In the first few days of hospitalization, he experienced a cardiac arrhythmia ending in asystole, from which he was promptly resuscitated, and a period of nonconvulsive status epilepticus that terminated with administration of fosphenytoin. He slowly lapsed into a permanent coma and developed choreoathetosis, which persisted until his death 8 weeks later.
Several factors may have contributed to this patient’s death. In addition to the olanzapine overdose, he developed a brief cardiac arrest with possible cerebral anoxia and hours of nonconvulsive status epilepticus. Although cerebral anoxia cannot be ruled out as a contributor, there was no evidence that the hypoxia was severe. Mr. A developed the cardiac arrhythmia and arrest in the intensive care unit with continuous cardiac monitoring and immediate resuscitation once the arrhythmia was recognized. Pulse oximetry before and after the arrest and measurement of arterial blood gases afterward never demonstrated findings of severe hypoxia. Repeat EEGs after the arrest never showed the burst suppression activity typically seen in severe cerebral hypoxia. The autopsy did not reveal evidence of global subacute ischemia in the cerebral cortex, striatum, or hippocampus, which would have been expected if the cardiac arrest at admission had been the cause of the coma.
Nonconvulsive status epilepticus is different from the more common convulsive status epilepticus (19). With increased diagnostic awareness and improved methods of EEG detection, nonconvulsive status epilepticus now represents about 20% of the cases of status epilepticus (19–21). The absence form of nonconvulsive status epilepticus is characterized by altered mental status (confusion, stupor, or coma), and typical clinical manifestations include prolonged repetitive eye blinking or isolated myoclonic jerks, mainly of the face (19–21). A less common form of nonconvulsive status epilepticus, often called complex partial status epilepticus, may by manifested by confusion, blinking, automatisms, and bizarre and fluctuating behavior (19, 21). Nonconvulsive status epilepticus is not associated with convulsive limb movements, hyperthermia, acidosis, hyperkalemia, pulmonary compromise, or cardiovascular collapse, as seen in convulsive status epilepticus. It may occur de novo or be associated with prior epilepsy, hypoxia, benzodiazepine withdrawal, brain tumor, CNS infection, head trauma, or stroke (19–21).
Nonconvulsive status epilepticus often persists for hours or days before the diagnosis is made. The EEG usually establishes the diagnosis and demonstrates epileptiform spike or sharp-wave discharges, a rhythmic appearance, and a discharge frequency of at least 1 Hz, which persist for 30 minutes or longer (19–21). Results of neuroimaging are usually normal but may demonstrate preexisting cerebral pathology (22). Abnormalities in the basal ganglia have not been seen. Treatment is with anticonvulsants. The prognosis for nonconvulsive status epilepticus is much better than that for convulsive status epilepticus. Most patients make a recovery that primarily relates to the severity of the underlying cause (19, 21). Limited brain histological studies have not identified a characteristic pathology (21). Thus, in Mr. A, the episode of nonconvulsive status epilepticus was not felt to be the cause of his persisting coma and choreoathetosis or basal ganglia pathology.
The neuroleptic malignant syndrome is a recognized adverse effect of olanzapine (23) and can cause death with brain pathology (24). However, our patient did not have the clinical features of fever, marked limb rigidity, and severe dystonic posturing that are usually seen. In favor of this possibility is that we do not have another explanation for the mildly elevated white blood cell count and serum creatinine kinase level on admission. The cardiac resuscitation was felt to account for the subsequent creatinine kinase elevation, which was transient.
Mr. A likely consumed 750 mg of olanzapine, resulting in a peak serum olanzapine level of 464.3 ng/ml. This peak level is similar to other blood levels in fatal overdoses (15, 16, 25–27). The manufacturer has received reports of fatalities from acute ingestion of as little as 450 mg and survival after ingestion of 1500 mg (10).
Our patient developed many of the recognized serious adverse effects, including cardiac arrhythmia, coma, nonconvulsive status epilepticus, and a persistent choreoathetosis with elements of dystonia. Except for the persistent choreoathetosis, similar adverse effects have been reported to occur occasionally with other atypical antipsychotics.
This patient offered the unusual opportunity to explore possible pathophysiologic mechanisms that might have contributed to the complications. The highest serum level of olanzapine was 18 times the median therapeutic serum value of 26 ng/ml for 1,653 patients (26). The elimination half-life of 52.5 hours in our patient was above the range of 27–39 hours for therapeutic doses in one report (28) and above the mean of 30 hours given in a review (3) but fell within the range of 21–54 hours in the review.
It is intriguing that the CSF olanzapine level was only about 1 ng/ml. A lack of olanzapine in CSF in fatal overdoses has been previously reported (15, 27). Since olanzapine is highly bound to plasma proteins, it is possible that little free olanzapine is available to cross the blood-CSF barrier (3). However, in one autopsy study of a fatal olanzapine overdose, the blood contained 400 ng/ml, the caudate and putamen contained 390 ng/g, but the CSF had undetectable levels (15). Studies of rats that were administered olanzapine by injection or gavage either once or for 15 consecutive days showed that olanzapine distributed widely in the brain at concentrations several times above the plasma level (29). Together these observations suggest that olanzapine crosses the blood-brain barrier better than the blood-CSF barrier or that olanzapine is rapidly removed from CSF.
Plasma levels of olanzapine metabolites reported by the manufacturer are typically half those of the parent drug (3). In our patient, the glucuronide olanzapine levels were initially twice as high as the level of the parent drug but were cleared in an expected time course. Since these metabolites are not biologically active at therapeutic levels (3), this information suggests they were unlikely to have contributed much to the neurologic dysfunction.
Brain histology findings for patients dying of olanzapine overdose usually have been unremarkable, but most of the autopsied patients died within hours to a few days of overdose (15–17). Our patient died 8 weeks after overdose and demonstrated gliosis in the basal ganglia without a corresponding loss of neurons. Areas of brain necrosis were not identified. Focal eosinophilic degeneration of neurons seen in sections of the neocortex and hippocampus is a common finding consistent with terminal hypoxia. The loss of Purkinje cells in the cerebellum is a nonspecific finding. One other case of olanzapine overdose demonstrated softening and necrosis of the caudate nuclei at autopsy (5).
Mr. A’s basal ganglia gliosis corresponds to the area in the MRI scan that was abnormal while he was alive. The presence of T2-weighted lesions without T1-weighted MRI abnormalities was consistent with the lack of frank basal ganglia necrosis found at autopsy. In summary, the gross and microscopic analyses of the brain suggest that the basal ganglia abnormalities were associated with the olanzapine overdose.
The choreoathetosis seen in our patient is compatible with basal ganglia dysfunction (30). Clinically, the choreoathetosis resembled the most common dyskinesia seen in patients receiving excessive levodopa or dopamine agonist medication, particularly individuals with Parkinson’s disease (31). In spite of the high olanzapine blood levels, Mr. A never developed rigidity or bradykinesia suggestive of Parkinson’s disease. This raises the possibility of the presence of excess dopamine in the striatum. In support of this possibility, an in vivo microdialysis study in experimental animals indicated that olanzapine increased tissue levels of dopamine by 278% in the striatum (32). In addition, in experimental studies olanzapine did not block and slightly enhanced the spontaneously firing A9 dopamine neurons, which project from the substantia nigra to motor areas of the striatum (33). In addition to olanzapine, phenytoin rarely also induces dyskinesias, including choreoathetosis (34, 35), and so it is possible that this drug contributed to the choreoathetosis.
Management of Mr. A’s case was also complicated by excessive secretion of saliva, which necessitated intubation to protect his airway. Hypersalivation from olanzapine has been previously reported at therapeutic doses (6).
Presently there is no definitive treatment or antidote for olanzapine overdose to reverse its pharmacologic effects or to enhance the drug’s elimination. Current management consists of early administration of activated charcoal to reduce the bioavailability of olanzapine, electrocardiographic monitoring for cardiac arrhythmias, and control of the airway with intubation if necessary (10). If hypotension develops, administration of intravenous fluids is helpful. Use of beta-agonist drugs should be avoided. Since 93% of olanzapine is bound to serum proteins (15), hemodialysis has little effect in lowering plasma drug concentrations. Although we were able to stop the seizures with therapeutic levels of fosphenytoin, we were unable to control the choreoathetosis. Better specific treatment methods for olanzapine overdoses are needed. If a patient is known to be suicidal, caution should be used when determining the number of olanzapine pills to be given to the patient at one time.
 |
 |
Received Jan. 24, 2004; revisions received May 25, and Sept. 2, 2004; accepted Sept. 10, 2004. From the Neurology and Radiology Services, New Mexico VA Health Care System; the Departments of Neurology, Neuroscience, Pathology, and Radiology, University of New Mexico, Albuquerque; the New Mexico Poison and Drug Information Center, Albuquerque; and Bioanalytical Systems, West Lafayette, Ind. Address correspondence and reprint requests to Dr. Davis, Neurology Service, New Mexico VA Health Care System, 1501 San Pedro Drive S.E., Albuquerque, NM 87108. The authors thank Eli Lilly and Company for prompt responses to questions and for assistance in obtaining the blood and CSF olanzapine levels, Dr. Cindi Starkey at the University of New Mexico for help in the postmortem evaluation, and Ms. Michelle Williams of the New Mexico VA Health Care System for technical assistance in obtaining blood samples.
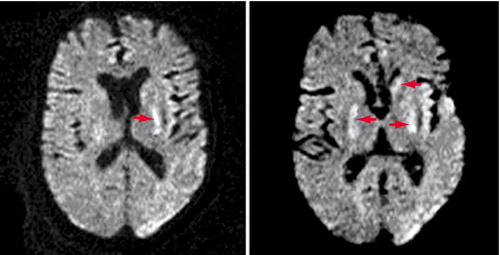
Figure 1. Consecutive Axial Diffusion-Weighted MRI Images for Mr. A in Week 6 of Hospitalization for Olanzapine Overdoseaa
Arrows point to focal areas of restricted diffusion in both the medial putaminis/posterior globus pallida and the left caudate head. Asymmetrical susceptibility artifacts with accompanying distortions project over the lateral left occipital lobe and left temporal operculum.
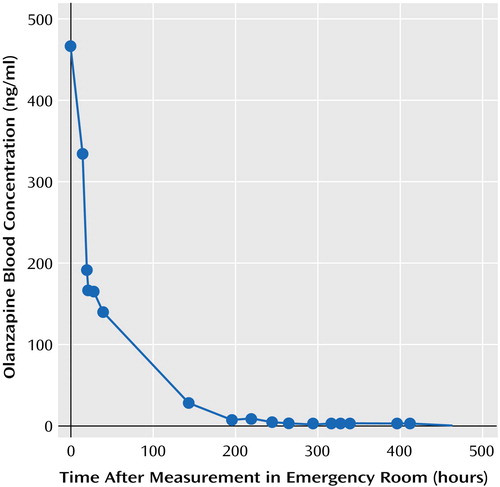
Figure 2. Olanzapine Blood Levels for Mr. A During Hospitalization for Olanzapine Overdose
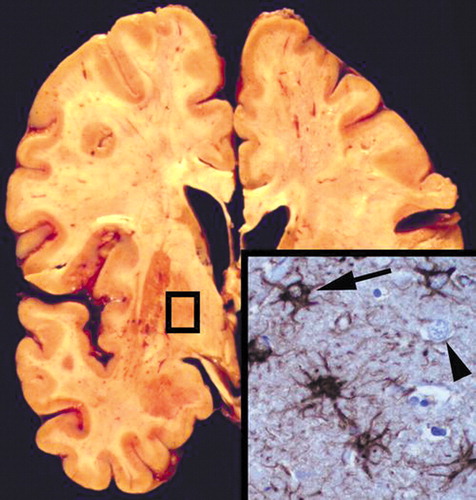
Figure 3. Coronal Section of Mr. A’s Brain, at the Level of the Globus Pallidus, After Death From Olanzapine Overdoseaa
The section is grossly normal without cavitation or infarcts but has excess cellularity (inset) with many reactive astrocytes (inset, arrow) and intact neurons (inset, arrowhead). Inset: antibody to glial fibrillary acidic protein with hematoxylin counterstain; original magnification, ×400.
1. Mulligan K: Antipsychotic comparison finds surprising result. Psychiatr News, July 4, 2003, pp 14, 38Google Scholar
2. Bymaster FP, Perry KW, Nelson DL, Wong DT, Rassmussen K, Moore NA, Calligaro DO: Olanzapine: a basic science update. Br J Psychiatry 1999; 174(suppl 37):36–40Google Scholar
3. Bever KA, Perry PJ: Olanzapine: a serotonin-dopamine-receptor antagonist for antipsychotic therapy. Am J Health Syst Pharm 1998; 55:1003–1016Crossref, Medline, Google Scholar
4. Beasley CM, Tollefson GD, Tran PV: Safety of olanzapine. J Clin Psychiatry 1997; 58(suppl 10):13–17Google Scholar
5. Wyderski RJ, Starrett WG, Abou-Saif A: Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother 1999; 33:787–789Crossref, Medline, Google Scholar
6. Perkins DO, McClure RK: Hypersalivation coincident with olanzapine treatment (letter). Am J Psychiatry 1998; 155:993–994Link, Google Scholar
7. Sheitman BB, Lindgren JC, Early J, Sved M: High-dose olanzapine for treatment-refractory schizophrenia (letter). Am J Psychiatry 1997; 154:1626Link, Google Scholar
8. Cohen LG, Fatalo A, Thompson BT, Di Centes Bergerson G, Flood JG, Poupolo PR: Olanzapine overdose with serum concentrations. Ann Emerg Med 1999; 34:275–278Crossref, Medline, Google Scholar
9. Chambers RA, Caracansi A, Weiss G: Olanzapine overdose cause of acute extrapyramidal symptoms (letter). Am J Psychiatry 1998; 155:1630–1631Link, Google Scholar
10. Zyprexa—Overdose Safety Profile. Indianapolis, Eli Lilly and Co, 2003Google Scholar
11. Sernyak MJ, Leslie DL, Alarcon RD, Losonczy MF, Rosenheck R: Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry 2002; 159:561–566Link, Google Scholar
12. Litovitz T, Klein-Schwartz W, White S, Cobaugh DJ, Youniss J, Omslaer J, Drab A, Benson BE: 2000 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 2001; 19:337–395Crossref, Medline, Google Scholar
13. Litovitz T, Klein-Schwartz W, Rogers GC, Cobaugh DJ, Youniss J, Omslaer J, Drab A, May ME, Woolf AD, Benson BE: 2001 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 2002; 20:391–452Crossref, Medline, Google Scholar
14. Watson WA, Litovitz T, Rogers GC, Klein-Schwartz W, Youniss J, Omslaer J, Rose SR, Borys D, May ME: 2002 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 2003; 21:353–421Crossref, Medline, Google Scholar
15. Merrick TC, Felo JA, Jenkins AJ: Tissue distribution of olanzapine in a postmortem case. Am J Forensic Med Pathol 2001; 22:270–274Crossref, Medline, Google Scholar
16. Gerber JE, Cawthon B: Overdose and death with olanzapine: two case reports. Am J Forensic Med Pathol 2000; 21:249–251Crossref, Medline, Google Scholar
17. Stephens BG, Coleman DE, Baselt RC: Olanzapine-related fatality. J Forensic Sci 1998; 43:1252–1253Medline, Google Scholar
18. Catlow JT, Barton RD, Clemens M, Gillespie TA, Goodwin M, Swanson SP: Analysis of olanzapine in human plasma utilizing reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl 1995; 668:85–90Crossref, Medline, Google Scholar
19. Dunne JW, Summers QA, Stewart-Wynne EG: Non-convulsive status epilepticus: a prospective study in an adult general hospital. Q J Med 1987; 238:117–126Google Scholar
20. Towne AR, Waterhouse EJ, Boggs JG, Garnett LK, Brown AJ, Smith JR Jr, DeLorenzo RJ: Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology 2000; 54:340–345Crossref, Medline, Google Scholar
21. Drislane FW: Evidence against permanent neurologic damage from nonconvulsive status epilepticus. J Clin Neurophysiol 1999; 16:323–331Crossref, Medline, Google Scholar
22. Sperling MR, Wilson G, Engel J, Babb TL, Phelps M, Bradley W: Magnetic resonance imaging in intractable partial epilepsy: correlative studies. Ann Neurol 1986; 20:57–62Crossref, Medline, Google Scholar
23. Kogoj A, Velikonja I: Olanzapine induced neuroleptic malignant syndrome—a case review. Hum Psychopharmacol Clin Exp 2003; 18:301–309Crossref, Medline, Google Scholar
24. Adnet P, Lestavel P, Krivosic-Horber R: Neuroleptic malignant syndrome. Br J Anaesth 2000; 85:129–135Crossref, Medline, Google Scholar
25. Elian AA: Fatal overdose of olanzapine. Forensic Sci Int 1998; 16:231–235Crossref, Google Scholar
26. Robertson MD, McMullin MM: Olanzapine concentrations in clinical serum and postmortem blood specimens—when does therapeutic become toxic? J Forensic Sci 2000; 45:418–421Medline, Google Scholar
27. Jenkins AJ, Sarconi KM, Raaf HN: Determination of olanzapine in a postmortem case. J Anal Toxicol 1998; 22:605–609Crossref, Medline, Google Scholar
28. Fulton B, Goa KL: Olanzapine: a review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs 1997; 53:282–298Crossref, Google Scholar
29. Aravagiri M, Teper Y, Marder SR: Pharmacokinetics and tissue distribution of olanzapine in rats. Biopharm Drug Dispos 1999; 20:369–377Crossref, Medline, Google Scholar
30. Filion M: Physiologic basis of dyskinesia. Ann Neurol 2000; 47(suppl 1):S35-S41Google Scholar
31. Fahn S: The spectrum of levodopa-induced dyskinesias. Ann Neurol 2000; 47(suppl 1):S2-S11Google Scholar
32. Li XM, Perry KW, Wong DT, Bymaster FP: Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology (Berl) 1998; 136:153–161Crossref, Medline, Google Scholar
33. Stockton ME, Rassmussen K: Electrophysiological effects of olanzapine, a novel atypical antipsychotic, on A9 and A10 dopamine neurons. Neuropsychopharmacology 1996; 14:97–104Crossref, Medline, Google Scholar
34. Montenegro MA, Scontoni AE, Cendes F: Dyskinesia induced by phenytoin. Arq Neuropsiquiatr 2000; 57:356–360Crossref, Google Scholar
35. Saito Y, Oguni H, Awaya Y, Hayashi K, Osawa M: Phenytoin-induced choreoathetosis in patients with severe myoclonic epilepsy in infancy. Neuropediatrics 2001; 32:231–235Crossref, Medline, Google Scholar



