A Videotape Intervention to Enhance the Informed Consent Process for Medical and Psychiatric Treatment Research
Abstract
OBJECTIVE: This study evaluated a brief educational video designed to enhance the informed consent process for people with serious mental and medical illnesses who are considering participating in treatment research. METHOD: Individuals with schizophrenia who were being recruited for ongoing clinical trials, medical patients without self-reported psychiatric comorbidity, and university undergraduates were randomly assigned to view either a highly structured instructional videotape about the consent process in treatment research or a control videotape that presented only general information about bioethical issues in human research. Knowledge about informed consent was measured before and after viewing. RESULTS: Viewing the experimental videotape resulted in larger gains in knowledge about informed consent. Standardized effect sizes were large in all groups. CONCLUSIONS: The videotape was thus an effective teaching tool across diverse populations, ranging from individuals with severe chronic mental illness to university undergraduates.
Concerns have been raised over the capacity of mentally ill patients to understand the risks and benefits of participating in clinical trials (1). There has been evidence for decades that medical patients may also have difficulty understanding informed consent information. Cassileth and colleagues (2) found that only 60% of 200 cancer patients participating in radiation, surgery, or chemotherapy recalled the nature and purpose of the procedure as little as a day after signing the consent, and most could not recall even one risk or benefit. Many misunderstood the purpose of the informed consent process as being for the physician’s protection. In previous work, we developed simple questionnaires designed to enhance and assess patients’ understanding of specific research protocols (3–5). This article describes a brief videotape teaching intervention that was designed as part of a larger study of the consent process. The videotape program was designed to enhance the consent process for medical and psychiatric treatment. Viewed by prospective research volunteers before the consent session, it alerts them to their rights as participants, informs them of the key requisite elements of informed consent, models active participation in the consent session itself, and discusses good decision making.
Method
Schizophrenia patients (N=83; age: mean=37.2 years, SD=13.9; education: mean=13.2 years, SD=1.7; 82% men) who were considering participation in one of 10 randomized clinical trials (most involving comparisons of medications) were recruited for this project at the West Los Angeles Veterans Administration (VA) Healthcare Center and the Aftercare Program of the University of California at Los Angeles. Medical patients without self-reported comorbid psychiatric illness (age: mean=59.1 years, SD=12.9; education: mean=14.1 years, SD=2.2; 82% men) were recruited from the West Los Angeles VA’s diabetes, nutrition, wellness, women’s, and outpatient clinics. University undergraduates without obvious medical, cognitive, or psychiatric problems (age: mean=21.4 years, SD=7.2; years of education: mean=13.1, SD=1.2; 40% men) volunteered to fulfill a requirement of a lower division psychology course. All subjects gave informed consent to participate. Although the groups obviously differed in age and gender, they were surprisingly similar in educational level. The high percentage of men among the patients was a natural consequence of the heavy reliance on VA clinical research settings.
The experimental video addresses three areas: 1) optimal behavior during the recruitment session, 2) the content that the informed consent session covers, and 3) good decision making. The videotape repeatedly emphasizes the need for the patient to be active, open about any failure to understand, and assertive in seeking information. Brief vignettes throughout the program show two professional actors playing a doctor/recruiter and a patient reviewing the major consent topics that are mandated by our institutional review boards, including the purpose of the study, the benefits, the risks, the voluntariness, and so on. The vignettes explain what each topic means and emphasize that it is the researcher’s responsibility to present each topic during the session. A final section emphasizes the need to consider pros and cons and to weigh costs and benefits of participation in the decision-making process. The videotape counsels that the patient may take his or her time in deciding and may consult with family, friends, or other advisors. Each point is presented in three modalities—text bullets, voiceover narration, and brief enacted vignettes. It is important to stress that the experimental videotape was deliberately written to be generic and applicable to a wide range of conditions, protocols, and treatments. Nothing in the content identifies whether the “patient” depicted is mentally or physically ill or hints at the nature of his condition or the study for which he is being recruited. The patient depicted is a Caucasian man who is probably in his 30s. The physician recruiter is an African American woman who is probably also in her mid-to-late 30s or early 40s. The control program is an educational presentation of general information about the history of societal concern about human subject research and regulatory mechanisms such as institutional review boards. It notes the importance that society places on protecting human subjects, but it primarily emphasizes the role of institutional protections, such as institutional review boards and government agencies, and it gives no specific information about the nature, process, or content of the consent session or of the responsibilities of either researcher or prospective participant. Both videotapes have the same “look and feel,” are 16–18 minutes long, and are written at a fifth-grade reading level. Most viewers perceive both as “educational.” Both are generic and suitable for general use in virtually any clinical research in medicine or psychiatry.
The subjects were randomly assigned to view one of the video programs. Knowledge about the consent process was measured before and after the viewing with an 80-item quiz covering key elements of the informed consent process written for this project (Assessment of Informed Consent Issues, http://www.npistat. com/aici.pdf). Coefficient alpha (reliability) of this measure was 0.90 in the schizophrenia group and 0.85 in both the student and medical groups. The schizophrenia participants also were evaluated with the Brief Psychiatric Rating Scale (BPRS) (6).
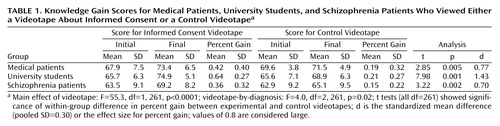
The primary measure of knowledge gain was percent improvement calculated by dividing the raw change by the total possible improvement. Thus, a score of 100% indicates a perfect final score, regardless of the initial score, and a score of 50% indicates that the subject got half of the items originally missed correct after viewing. A simple analysis of change yielded significant interactions of change by baseline (i.e., a larger change for those starting lower). This was considered a measurement artifact, and conversion to the percent gain metric removed all such interactions. Initial scores were analyzed by using one-way analysis of variance, and the knowledge change data were analyzed with a three-by-two (subject group-by-videotape condition) analysis of covariance of percent knowledge gain by using baseline knowledge score as a covariate. Table 1 presents initial and final means, standard deviations, mean percent improvement, within-group t tests and p values, and a standardized “effect size” index (d) computed by dividing the mean difference by the pooled standard deviation (7). In the schizophrenia group, Pearson’s correlations were computed between some BPRS items and initial and percent gain scores on the 80-item informed consent knowledge quiz.
Results
Table 1 summarizes the data. Initial knowledge scores differed (F=8.6, df=2, 265, p<0.001; all pairwise differences were significant by t test); the VA medical patients had the highest baseline scores, and the schizophrenia subjects had the lowest. There was a significant main effect of videotape condition, indicating far more improvement in knowledge for those viewing the experimental videotape in each subject group (F=55.3, df=1, 261, p<0.0001). Although the effect was small, a significant interaction of videotape condition by group (F=4.0, df=2, 261, p=0.02) indicated that the student group had a larger videotape effect than either patient group. Within-sample t tests were significant in all groups, and standardized effect sizes were statistically large to very large in all groups. In the schizophrenia group, higher scores on BPRS conceptual disorganization were moderately correlated with lower baseline (before viewing) test scores on the informed consent quiz (r=–0.38, df=80, p<0.001). However, conceptual disorganization did not correlate meaningfully with percent gain in the experimental (r=0.06, df=41, n.s.) or control videotape groups (r=–0.14, df=35, n.s.) when the baseline score was statistically controlled. Similarly, BPRS positive and negative symptom cluster scores and the BPRS total score were all uncorrelated with percent gain in the experimental videotape group (r ranged from –0.16 to 0.18, all df=41, all n.s.), suggesting that knowledge gains associated with viewing the experimental videotape were relatively independent of symptom level, at least within the range in this study group.
Conclusions
We believe that our brief videotape is a valid teaching tool for a broad range of prospective clinical research participants, including those with schizophrenia. This is consistent with Carpenter et al. (8), who reported that individuals with schizophrenia can benefit from educational interventions during the informed consent process across a range of symptom severity. This study also echoes our previous finding that schizophrenia patients with more severe conceptual disorganization may be most in need of assistance with the consent process (3). Current symptom severity did seem to matter, but the participating clinical trials involved maintenance treatments of stabilized schizophrenia patients. Patients who were acutely psychotic were screened out by a clinician before the issue of participating in a maintenance study was even broached. Our videotape program may thus have applicability to the recruitment of persons with very varied cognitive abilities for medical and psychiatric treatment research in diverse research settings.
 |
Presented in part at the 154th annual meeting of the American Psychiatric Association, New Orleans, May 5–10, 2001; the annual meeting of the Society for Biological Psychiatry, San Francisco, May 17, 2003; the International Congress on Schizophrenia Research, Whistler, B.C., Canada, May 2001; and the 40th annual meeting of the American College of Neuropsychopharmacology, Hawaii, December 2001. Received Feb. 21, 2004; revision received April 17, 2004; accepted May 10, 2004. From the Department of Psychiatry and Biobehavioral Sciences, the David Geffen School of Medicine, University of California at Los Angeles; the Department of Psychiatry, Greater Los Angeles VA Healthcare System; the Department of Psychology, California State University at Northridge, Northridge, Calif. Address correspondence and reprint requests to Dr. Mintz, Greater Los Angeles VA Healthcare System, 11301 Wilshire Blvd. (116AR), Los Angeles, CA 90073; [email protected] (e-mail). The authors thank William C. Wirshing, Mark Frye, Lori Altshuler, Michael Green, Stephen Marder, Keith Nuechterlein, Joseph Pierre, and John Kobashigawa for referring study participants; Jennifer Boyd, Robert Kern, R.P. Liberman, C.J. Wallace, the National Alliance for the Mentally Ill, the National Alliance for Research on Schizophrenia and Depression, Lois Mintz, and William C. Wirshing for contributing to the development of the video program; Joseph Ventura and Helen Lu for testing and recruiting patients; Sun Hwang for statistical support; and Seth Resnick and Shirley Mena for assisting with manuscript preparation. Supported by NIMH grant R01-MH-68100-02 to Jim Mintz.
1. Bonnie R: Research with cognitively impaired subjects: unfinished business in the regulation of human research. Arch Gen Psychiatry 1997; 54:105–111Crossref, Medline, Google Scholar
2. Cassileth BR, Zupkis RV, Sutton-Smith K, March V: Informed consent—why are its goals imperfectly realized? N Engl J Med 1980; 302:896–900Crossref, Medline, Google Scholar
3. Wirshing DA, Wirshing WC, Marder SR, Liberman RP, Mintz J: Informed consent: assessment of comprehension. Am J Psychiatry 1998; 155:1508–1511Link, Google Scholar
4. Wirshing W: In a perfect world none of this would concern us. J California Alliance for the Mentally Ill 1994; 5:30Medline, Google Scholar
5. Grabowski J, O’Brien CP, Mintz J: Increasing the likelihood that consent is informed. J Appl Behav Anal 1979; 12:283–284Medline, Google Scholar
6. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertaining and scaling. Psychopharmacol Bull 1988; 24:97–99Google Scholar
7. Cohen J: Statistical Power for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1998Google Scholar
8. Carpenter WT Jr, Gold JM, Lahti AC, Queern CA, Conley RR, Bartko JJ, Kovnick J, Appelbaum PS: Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry 2000; 57:533–538Crossref, Medline, Google Scholar



