Associated Alterations of Striatal Dopamine D2/D3 Receptor and Transporter Binding in Drug-Naive Patients With Schizophrenia: A Dual-Isotope SPECT Study
Abstract
OBJECTIVE: In vivo imaging studies have revealed deregulated presynaptic or postsynaptic function of the midbrain dopaminergic system in patients with schizophrenia. To further delineate the neuropathological involvement of the presynaptic and postsynaptic dopamine neurons in schizophrenia, the authors examined brain D2-family receptor and dopamine transporter binding simultaneously in patients with drug-naive schizophrenia using the dual-isotope single photon emission computed tomography (SPECT) imaging technique. METHOD: Eleven patients with schizophrenia and 12 healthy comparison subjects were recruited. Striatal dopamine D2/D3 receptor was measured with SPECT and [123I]iodobenzamide ([123I]IBZM), while dopamine transporter was measured with SPECT and [99mTc]TRODAT-1. RESULTS: Striatal D2/D3 receptor and dopamine transporter binding were unaltered in these drug-naive patients with schizophrenia. Nonetheless, D2/D3 receptor binding measures were positively correlated with dopamine transporter binding measures in these patients but not in the comparison subjects. CONCLUSIONS: The associated presynaptic and postsynaptic disturbances of midbrain dopamine neurons could be clinically relevant in drug-naive patients with schizophrenia.
Postsynaptic dopamine hypersensitivity in patients with schizophrenia has been reported (1) and supported by the amphetamine challenge test in drug-naive patients with schizophrenia (2). Conjunct alterations in presynaptic dopamine transporters and presynaptic and postsynaptic dopamine D2/D3 receptors could be involved in such dopaminergic disturbances in schizophrenia. Simultaneous detection of dopamine transporter and D2/D3 receptors in vivo is available by employing two specific isotopes with different energy windows to label these membrane targets (3). Therefore, dual-isotope single photon emission computed tomography (SPECT) was used to examine and quantify the dopamine transporter and D2/D3 receptors simultaneously in drug-naive patients with schizophrenia.
Method
Eleven drug-naive patients who fulfilled DSM-IV criteria for schizophrenia were recruited. Exclusion criteria consisted of any of the following conditions: any medical or neurological diseases, any previous use of antipsychotics, ECT, lithium treatment, history of alcohol or substance dependence, or head injury. The research protocol was approved by the Ethical Committee for Human Research at National Cheng Kung University Medical Center.
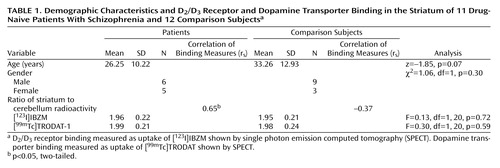
The patients’ ages ranged from 17 to 46 years; their mean age was 25.4 (SD=10.2). Seven had paranoid schizophrenia, three had disorganized schizophrenia, and one had undifferentiated schizophrenia. Their mean duration of illness was approximately 1.3 years (SD=2.2). Their mean score on the Positive and Negative Syndrome Scale (4) was 63.8 (SD=10.8, range=47–80).
The ages of the 12 healthy volunteers ranged from 20 to 53; their mean age was 33.3 (SD=12.9). They were interviewed with the Chinese version of the Mini International Neuropsychiatric Interview (5) to exclude individuals with mental illnesses. They did not have a history of substantial physical illness or any first-degree relative with a history of schizophrenia.
A single bolus injection of 740 MBq (20 mCi) [99mTc]TRODAT-1 (a radiolabeled form of tropane derivative for selectively labeling dopamine transporter) was administered (6), followed 2 hours later by an injection of 185 MBq (5 mCi) of [123I]iodobenzamide ([123I]IBZM) for labeling D2/D3 receptors.
Each subject was in a quiet environment for about 10 minutes after we set the intravenous lines. Two sets of SPECT data were obtained, with an energy window of 15% centered on 140 keV for technetium-99m and set 10% asymmetrically with the lower bound at 159 keV for iodine-123. Imaging of [99mTc]TRODAT-1 was initiated approximately 240 minutes after injection of [99mTc]TRODAT-1, and imaging of [123I]IBZM was initiated 120 minutes after injection. The method of acquiring the SPECT images was similar to that reported in our previous study (7).
An experienced nuclear medicine specialist who was blind to the patients’ clinical data drew the regions of interest manually on the basis of the individual magnetic resonance images (GE Sigma CV-I, 1.5 T, General Electric Systems, Milwaukee). The ratio of the radioactivity in the striatum to that in the cerebellum was derived by dividing the average counts per pixel in the striatum by the average counts per pixel in the cerebellum.
Mann-Whitney U test (two-tailed), chi-square test, and one-way analysis of covariance (ANCOVA) controlling for age were employed to examine the group differences. Spearman’s correlation (rs) and partial correlation (r) controlling for age were used to assess the relationship between the densities of dopamine transporter and D2/D3 receptors.
Results
ANCOVA showed that the patients and comparison subjects did not differ in the striatum-to-cerebellum radioactivity ratios of [123I]IBZM and [99mTc]TRODAT-1. The positive correlation between [123I]IBZM and [99mTc]TRODAT-1 striatum-to-cerebellum radioactivity ratios was evident in patients with schizophrenia (Table 1). After the age effect was partialled out, the marginal significance level still remained (r=0.62, df=8, p=0.06). However, there was no correlation between these two ratios in comparison subjects (r=–0.50, df=9, p=0.12) (Table 1). Neither the [123I]IBZM nor the [99mTc]TRODAT-1 binding measures correlated with the positive and negative symptom dimensions.
The patient group was further divided into those with high (≥2.0) and low (<2.0) TRODAT ratios. The group with a high TRODAT ratio demonstrated a slightly longer duration of illness from their first diagnosis than the low TRODAT ratio group (z=–1.46, p=0.14).
Discussion
Although a presynaptic dysfunction in the midbrain dopamine system has been speculated (8), we found that striatal dopamine transporter binding was not altered in drug-naive patients experiencing their first episode of schizophrenia. These findings are in parallel with the results of others (9–12). No correlations were obtained between dopamine transporter density and symptoms, a finding that is also supported by others (10, 12). Dopamine hypersensitivity has been postulated in schizophrenia for several decades. However, the results of D2/D3 receptor binding studies in drug-naive schizophrenic patients remain controversial. Laruelle et al. (13) demonstrated that competition by endogenous dopamine results in a 20%–30% underestimation of D2/D3 receptor density in vivo. This possibility may account for the inconsistent results in efforts to measure striatal presynaptic or postsynaptic membrane protein bindings.
In this study, we demonstrate for the first time to our knowledge that D2/D3 density is correlated with dopamine transporter binding in drug-naive patients with schizophrenia. There seems to be negative correlation between D2/D3 and dopamine transporter densities in healthy subjects, which is the opposite direction of the association in patients with schizophrenia. Combined with the results of amphetamine challenge test in patients with schizophrenia (2), the association between striatal D2/D3 and dopamine transporter densities may reflect a hypersensitivity and/or hyperfunction of the central dopaminergic system in patients with schizophrenia compared with healthy subjects. We suspect that D2/D3 receptor and dopamine transporter could be down-regulated in the early phase of disease development. Nonetheless, simultaneous decreases in D2/D3 receptor and dopamine transporter could be up-regulated by some unknown compensatory mechanisms as the disease progresses. Because of the limited number of patients in our study, our findings mandate further investigation.
 |
Received Oct. 9, 2003; revision received Dec. 18, 2003; accepted Jan. 9, 2004. From the Department of Psychiatry, Institute of Behavioral Medicine, Department of Nuclear Medicine, College of Medicine, National Cheng Kung University. Address reprint requests to Dr. Yang, Department of Psychiatry, College of Medicine, National Cheng Kung University, 138, Sheng Li Road, Tainan, Taiwan 704, ROC; [email protected] (e-mail). Supported in part by grants NI302, 90-NU-7-006-004, and 91-NU-7-006-002 from the Atomic Energy Council of Taiwan and grant NSC-91–2314-B-006-074 from the National Science Council of Taiwan. The authors thank Dr. Wei Jen Yao at the Department of Nuclear Medicine and Ms. Ching Lin Chu and Shu Chuan Lin at the Department of Psychiatry, National Cheng Kung University, and the research participants.
1. Owen F, Cross AJ, Crow TJ, Longden A, Poulter M, Riley GJ: Increased dopamine-receptor sensitivity in schizophrenia. Lancet 1978; 2:223–226Crossref, Medline, Google Scholar
2. Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D: Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 1997; 94:2569–2574Crossref, Medline, Google Scholar
3. Dresel SH, Kung MP, Huang XF, Plossl K, Hou C, Meegalla SK, Patselas G, Mu M, Saffer JR, Kung HF: Simultaneous SPECT studies of pre- and postsynaptic dopamine binding sites in baboons. J Nucl Med 1999; 40:660–666Medline, Google Scholar
4. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
5. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC: The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Google Scholar
6. Kung HF: Development of Tc-99m labeled tropanes: TRODAT-1, as a dopamine transporter imaging agent. Nucl Med Biol 2001; 28:505–508Crossref, Medline, Google Scholar
7. Yang YK, Chiu NT, Chen CC, Chen M, Yeh TL, Lee IH: Correlation between fine motor activity and striatal dopamine D2 receptor density in patients with schizophrenia and healthy controls. Psychiatry Res Neuroimaging 2003; 123:191–197Crossref, Medline, Google Scholar
8. Bannon MJ, Granneman JG, Kapatos G: The dopamine transporter: potential involvement in neuropsychiatric disorders, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 179–188Google Scholar
9. Hsiao MC, Lin KJ, Liu CY, Tzen KY, Yen FC: Dopamine transporter change in drug naive schizophrenia: an imaging study with 99mTc TRODAT-1. Schizophr Res 2003; 65:39–46Crossref, Medline, Google Scholar
10. Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, Peurasaari J, Räkköläinen V, Syvälahti E, Hietala J: Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry 2000; 157:269–271Link, Google Scholar
11. Laruelle M, Abi-Dargham A, van Dyck C, Gil R, D’Souza DC, Krystal J, Seibyl J, Baldwin R, Innis R: Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biol Psychiatry 2000; 47:371–379Crossref, Medline, Google Scholar
12. Lavalaye J, Linszen DH, Booij J, Dingemans PM, Reneman L, Habraken JB, Gersons BP, van Royen EA: Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophr Res 2001; 47:59–67Crossref, Medline, Google Scholar
13. Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, Baldwin RM, Kung HF, Charney DS, Hoffer PB, Innis RB, Bradberry CW: Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse 1997; 25:1–14Crossref, Medline, Google Scholar



