Pathology of Layer V Pyramidal Neurons in the Prefrontal Cortex of Patients With Schizophrenia
Abstract
OBJECTIVE: Morphological indications of abnormal circuitry have been detected in the prefrontal neuropil of patients with schizophrenia. The authors tested the hypothesis that schizophrenia is associated with smaller dendritic field size in layer V pyramidal neurons in the prefrontal cortex. METHOD: Tissue from area 10 with a mean postmortem interval of 5.7 hours was obtained from 15 subjects with chronic schizophrenia and 18 normal comparison subjects. After Golgi impregnation, basilar dendritic field size was estimated for layer V pyramidal neurons by ring intersection analysis. RESULTS: The schizophrenia subjects had 40% fewer total ring intersections per neuron than comparison subjects. Smaller basilar dendritic field size was evident in proximal and distal branches. CONCLUSIONS: These results indicate that abnormal dendritic outgrowth or maintenance contributes to reduced neuropil and prefrontal connectivity in schizophrenia. Short postmortem intervals and resulting high tissue quality suggest that these dystrophic changes reflect schizophrenia pathology rather than postmortem artifact.
Schizophrenic patients exhibit neuropsychological and physiological impairments in prefrontal cortical functioning (1). Studies revealing lower volumes of prefrontal gray matter and higher cell-packing density in pyramidal cell layers of areas 9 and 46 support the “reduced neuropil hypothesis” (2). These results may reflect less thalamocortical input, resulting in observed reductions in dendritic length and spine density in layer III pyramidal neurons (3–5).
Neuronal pathologies in schizophrenia also appear to involve less dopaminergic input to the deep layers of the prefrontal cortex (6). The targets of this input include pyramidal neurons in layer V, which are the primary source of subcortical output and have collateral feedback with pyramidal neurons in layer III (7). Loss of dopaminergic projections may be associated with dendritic atrophy similar to that observed in layer III. The density of layer V pyramidal cells in schizophrenic subjects is higher than normal in the frontopolar cortex (area 10) (8), where ultrastructural synaptic pathologies have also been detected (9). In the present study we investigated whether layer V pyramidal neurons in area 10 of patients with chronic schizophrenia exhibit quantitative morphological abnormalities.
Method
The University of Illinois institutional review board and the Mental Health Research Center in Moscow (Russia) approved the research, and consent from each family was obtained. Subject selection, diagnostic approach, and neuropathological examination have been described previously (10). The criteria for subject inclusion were a short postmortem interval and cardiovascular disease or pneumonia as the cause of death. The exclusion criteria were ischemic, degenerative, or metabolic disorders and a history of head trauma, brain neoplasm, or alcohol or drug abuse. The schizophrenic subjects included eight women and seven men with a mean age of 59.9 years (SD=17.0) and a mean postmortem interval of 6.0 hours (SD=1.6). They were inpatients in Moscow psychiatric hospitals and had been diagnosed before admission or in the hospitals, in which they resided for a mean of 18 years. ICD-10 diagnoses of schizophrenia for all 15 patients were confirmed independently by two senior Russian psychiatrists (including D.O.) using chart reviews and family interviews. The comparison subjects included two women and 16 men with a mean age of 50.6 years (SD=11.6) and a postmortem interval of 5.4 hours (SD=0.9). They were inpatients or outpatients from Moscow community hospitals and had no history of psychiatric disorders, as determined from medical records and family interviews. (A table describing the subjects can be obtained from Dr. Greenough on request.)
Experienced neuropathologists (including V.V.) performed the autopsies, removing tissue from area 10 (frontal pole, inferior frontal sulcus) of the left hemisphere. Several subcortical regions and cortical areas 10, 38, 17, and 1 were examined for the exclusion criteria (listed in the preceding paragraph) by using the following histopathological stains: Nissl, hematoxylin-eosin, van Gieson, Bielschowsky, and Congo red. Electron microscopy of adjacent blocks showed minimal postmortem changes (9, 10). One subject was excluded for neuropathology (astrocytoma).
A Golgi-Kopsch modification (11) was used to impregnate blocks from area 10, and staining quality and neuron inclusion criteria were assessed without knowledge of diagnosis. Layer V pyramidal neurons with at least two bifurcating basilar dendrites and full impregnation of intact soma and distal dendrites were traced with a camera lucida at 750×. Nine or 10 neurons per subject were randomly selected from multiple fields of view within several 120-μm-thick sections. Three investigators (including I.M.K. and A.W.G.), blind to diagnosis, traced the neurons and counted the intersections between dendrites and a concentric ring overlay (12) to estimate total basilar dendritic field size. Interrater reliability, as indicated by the intraclass correlation coefficient for the three drawers across 14 random subjects, was 0.991 (13).
The mean number of ring intersections per neuron per subject was analyzed by using analysis of variance (ANOVA) (SAS version 8.02; Cary, N.C., SAS Institute), with age and diagnosis as main effects. Type 1 sums of squares were used to account for variance due to age before the effect of diagnosis was tested.
Results
Typical neurons from each group are displayed in Figure 1, which includes results for total ring intersections with basilar dendrites and their spatial distribution relative to the soma. The mean total number of ring intersections per neuron was 78.6 (SD=31.1) for the comparison subjects and 46.8 (SD=11.9) for the schizophrenic subjects. After accounting for age, ANOVA revealed a significant effect of diagnosis on this measure (Figure 1). When included in the model, age and diagnosis did not show a significant interaction. Four of the schizophrenia patients were older than any comparison subject, but analysis without these subjects still indicated a significant effect of diagnosis, as did analyses without the youngest subjects of both groups.
Discussion
The total basilar dendritic field size of prefrontal layer V pyramidal cells was 40% smaller in these patients with schizophrenia than in normal subjects. These findings are consistent with observations of less neuropil in the prefrontal cortex of patients with schizophrenia, including shorter basilar dendritic length (4, 5) and lower spine density (3, 4) in layer III pyramidal neurons as well as an initial report on layer V basilar dendrites (14). Other findings of ultrastructural synaptic abnormalities (9) and higher pyramidal cell-packing density in layers III (2) and V (8) are also consistent with our results. The reduced prefrontal neuropil may be associated with less dopaminergic innervation of the deep layers of the prefrontal cortex (6).
Although the comparison group had a skewed sex ratio, in the schizophrenia group there was no effect of sex on the number of ring intersections. When the men were analyzed separately (after accounting for age), the effect of diagnosis on ring intersections remained significant (F=9.83, df=2, 20, p=0.006). Prolonged medication may have contributed to reduced neuropil, but prior neuroleptic studies do not support this view (15), and medication history did not appear to correlate with dendritic field size within these subjects. This finding of less layer V pyramidal dendritic material in high-quality postmortem tissue, combined with previous reports, suggests that schizophrenia is associated with profound, multifocal disturbances of connectivity in the prefrontal cortex.
Presented in part at the 28th annual meeting of the Society for Neuroscience, Los Angeles, Nov. 7–12, 1998, and at the International Congress for Schizophrenia Research, Santa Fe, N.Mex., April 17–21, 1999. Received May 30, 2001; revisions received May 7, 2002, and March 27, 2003; accepted Aug. 28, 2003. From the Departments of Psychiatry and Psychology and the Beckman Institute, University of Illinois at Urbana-Champaign; and the Mental Health Research Center, Russian Academy of Medical Sciences, Moscow. Address reprint requests to Dr. Greenough, Beckman Institute, 405 North Mathews Ave., Urbana, IL 61801; [email protected] (e-mail). Funded by the McDonnell Foundation. The authors thank Abhay Laddu and members of the Laboratory of Clinical Neuropathology at the Mental Health Research Center in Moscow.
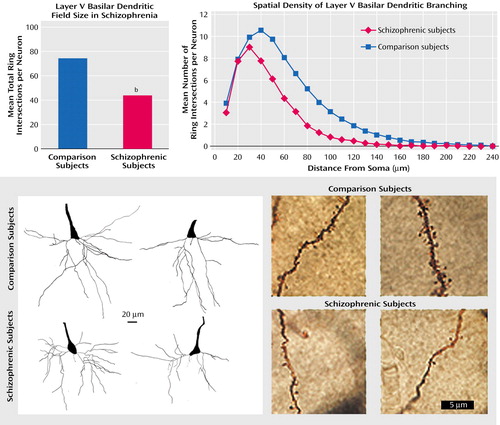
Figure 1. Smaller Dendritic Field Size of Pyramidal Neurons in the Prefrontal Cortex of 15 Patients With Schizophrenia Than in 18 Normal Comparison Subjectsa
aUpper left: total dendritic field size for comparison and schizophrenic subjects. Upper right: spatial distribution of concentric ring intersections: schizophrenic patients had fewer branches across proximal and distal dendrites. Lower left: pyramidal neurons from schizophrenic patients (bottom two neurons) had, on average, a 40% smaller basilar dendritic field size than those from comparison subjects (top two). Lower right: fully impregnated dendrites from comparison subjects (top two) and schizophrenic subjects (bottom two).
bDendritic field size was significantly smaller for schizophrenic subjects than for comparison subjects (F=9.31, df=2, 30, p=0.005).
1. Schultz SK, Andreasen NC: Schizophrenia. Lancet 1999; 353:1425–1430Crossref, Medline, Google Scholar
2. Selemon LD, Goldman-Rakic PS: The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Crossref, Medline, Google Scholar
3. Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR: Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry 1998; 65:446–453Crossref, Medline, Google Scholar
4. Glantz LA, Lewis DA: Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000; 57:65–73Crossref, Medline, Google Scholar
5. Kalus P, Muller TJ, Zuschratter W, Senitz D: The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport 2000; 11:3621–3625Crossref, Medline, Google Scholar
6. Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA: Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 1999; 156:1580–1589Link, Google Scholar
7. DeFelipe J, Farinas I: The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol 1992; 39:563–607Crossref, Medline, Google Scholar
8. Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL: Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 1991; 48:996–1001Crossref, Medline, Google Scholar
9. Uranova NA, Orlovskaya DD: Ultrastructural pathology of neuronal connectivity in postmortem brains of schizophrenic patients, in Annals of Psychiatry: Basic and Clinical Neurosciences, vol 6. Edited by Cacabelos R. Barcelona, Prous Science, 1996, pp 55–72Google Scholar
10. Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V: Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull 2001; 55:597–610Crossref, Medline, Google Scholar
11. Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT: Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet 2001; 98:161–167Crossref, Medline, Google Scholar
12. Sholl DA: Organization of the Cerebral Cortex. London, Methuen, 1956Google Scholar
13. Shrout PE, Fleiss JL: Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86:420–428Crossref, Medline, Google Scholar
14. Broadbelt K, Byne W, Jones LB: Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res 2002; 58:75–81Crossref, Medline, Google Scholar
15. Selemon LD, Lidow MS, Goldman-Rakic PS: Increased volume and glial density in primate prefrontal cortex associated with chronic antipsychotic drug exposure. Biol Psychiatry 1999; 46:161–172Crossref, Medline, Google Scholar



