Serotonin1A Receptors at the Axon Initial Segment of Prefrontal Pyramidal Neurons in Schizophrenia
Abstract
OBJECTIVE: Inhibition mediated by γ-aminobutyric acid at the axon initial segment of pyramidal neurons appears to be altered in the prefrontal cortex in schizophrenia. This study examined the densities and laminar distribution of axon initial segments labeled with an antibody against the serotonin1A (5-HT1A) receptor, which also mediates inhibitory regulation of pyramidal neurons, in subjects with schizophrenia. METHOD: The densities and laminar distribution of axon initial segments with 5-HT1A-like immunoreactivity were assessed in postmortem tissue from the prefrontal cortex (Brodmann’s area 46) of 14 matched triads of subjects with schizophrenia, subjects with major depressive disorder, and comparison subjects with no psychiatric disorder. RESULTS: The relative densities of the labeled axon initial segment in both the superficial and the deep cortical layers did not differ across the three subject groups. CONCLUSIONS: The findings do not support a role for altered serotonin transmission by means of the 5-HT1A receptor in dysfunction of prefrontal cortex pyramidal neurons in schizophrenia.
Dysfunction of the prefrontal cortex in schizophrenia is associated with alterations in excitatory pyramidal neurons and in inhibitory neurotransmitter systems that regulate pyramidal neuron activity. For example, subjects with schizophrenia exhibit alterations in both pre- and postsynaptic markers of γ-aminobutyric acid (GABA) neurotransmission at the axon initial segment of pyramidal neurons in the prefrontal cortex, the site of action potential generation (1, 2). It is interesting to note that the serotonin1A (5-HT1A) receptor has been reported to be highly concentrated at the axon initial segment of pyramidal neurons (3), where it mediates neuronal hyperpolarization by activating potassium channels (4). Postmortem autoradiographic studies have reported higher concentrations of 5-HT1A receptors in the prefrontal cortex, including Brodmann’s area 46, in subjects with schizophrenia (5), although a recent study that used positron emission tomography did not detect altered 5-HT1A receptor binding in the dorsolateral prefrontal cortex (6). However, these studies lacked the resolution required to identify 5-HT1A receptors localized to the axon initial segment of pyramidal neurons. Consequently, in this study, we sought to determine whether the density or laminar distribution of axon initial segments with 5-HT1A-like immunoreactivity is altered in the prefrontal cortex in subjects with schizophrenia and whether such changes are diagnostically specific.
Method
The 14 subject triads, each consisting of one subject with schizophrenia, one subject with major depressive disorder, and one comparison subject with no psychiatric disorder, examined in this study have been described previously (2). The subjects within the triads were matched for sex and matched as closely as possible for age and postmortem interval. Subject groups did not significantly differ in mean age, postmortem interval, or tissue storage time (2). After we obtained informed consent from the subjects’ next of kin, brain tissue was collected during autopsies conducted at the Allegheny County (Penn.) Coroner’s Office and handled in an identical fashion for each subject (i.e., 48-hour immersion fixation in a solution of 4% paraformaldehyde followed by cryoprotection and storage at –30° C) (2). A consensus diagnosis was obtained for each subject by an independent panel of clinicians (1).
Three tissue sections (40-μm thick) containing Brodmann’s area 46 of the prefrontal cortex from each subject were processed for immunocytochemistry (1) in a randomized block design (2) by using a rabbit polyclonal antibody (diluted to a concentration of 1:2000) raised against amino acids 170–186 in the second intracellular loop of the 5-HT1A receptor (7). Although the specificity of this antibody has been previously demonstrated (7), the pattern of labeling differs from that of antibodies directed against the third intracellular loop and we cannot exclude cross-reactivity with an unknown protein localized to the axon initial segment. Sections were mounted on coded slides, and the reaction product was intensified with osmium tetroxide followed by silver nitrate (8).
For each section, the axon initial segments with 5-HT1A-like immunoreactivity were randomly sampled in the superficial (layers 2–3a) and deep (layers 5–6) zones of the cortex by using Stereo Investigator fractionator software (MicroBrightField, Inc., Williston, Vt.), as previously described (1, 2). All quantification was conducted by one investigator (D.A.C.) who was blinded to the subject’s identifying number and diagnosis. Intrarater assessments of 5-HT1A-labeled axon initial segment identification resulted in an intraclass correlation coefficient of 0.99 (95% confidence interval=0.98–0.99).
Between-group comparisons were made by using analysis of covariance (ANCOVA), with age, sex, postmortem interval, and tissue storage time as covariates. The influences of sex, lifetime history of substance abuse, cause of death, and psychotropic drug exposure at the time of death on group differences, relative to the comparison group, in the density of 5-HT1A-labeled axon initial segments were also assessed by using ANCOVA.
Results
In brightfield photomicrographs, the 5-HT1A-labeled axon initial segments appeared as intensely immunoreactive vertical structures that tapered from the superficial to deep cortical layers and were located below the unlabeled cell bodies of pyramidal neurons (Figure 1). In all subjects, the density of the 5-HT1A-labeled axon initial segments appeared to be greater in the superficial than in the deep cortical layers, and layer 4 contained few labeled axon initial segments (Figure 1).
The mean density of 5-HT1A-labeled axon initial segments (measured in segments/mm2) did not differ between healthy comparison subjects, subjects with schizophrenia, and subjects with major depressive disorder in either the superficial zones (mean=784.1, SD=151.1; mean=827.2, SD=161.7; and mean=794.7, SD=197.6, respectively) or the deep zones (mean=456.9, SD=171.2; mean=515.0, SD=128.2; and mean=422.8, SD=143.4, respectively) of Brodmann’s area 46 (Figure 1). In addition, the difference in 5-HT1A-labeled axon initial segment density between individual subjects with schizophrenia or major depressive disorder and their matched comparison subjects did not differ in either the superficial or the deep cortical zones as a function of sex, lifetime history of substance abuse, cause of death, or psychotropic drug exposure at the time of death (F<0.84, df=1, 11, p>0.38).
Discussion
Due to the location of the axon initial segment near the site of action potential generation, inhibition at the axon initial segment plays a potent role in regulating the output of pyramidal neurons (9). Convergent findings indicate a lower level of GABA neurotransmission at the axon initial segment in patients with schizophrenia, relative to comparison subjects with no psychiatric disorder. For example, in Brodmann’s area 46 in the prefrontal cortex of subjects with schizophrenia, relative to comparison subjects with no psychiatric disorder, immunoreactivity for the GABA membrane transporter is lower in the axon terminals of chandelier cells, the subclass of GABA neurons that selectively target the axon initial segment of pyramidal neurons, whereas greater immunoreactivity is found for the α2 subunit of the GABAA receptor, which is preferentially localized to the pyramidal neuron axon initial segment (1, 2). In contrast, the findings of this study demonstrate that in the same subject cohorts, neither the relative density nor laminar distribution of axon initial segments with 5-HT1A-like immunoreactivity differed in the subjects with schizophrenia, relative to the comparison subjects with no psychiatric disorder or to the subjects with major depressive disorder. Because 5-HT1A receptors at other cellular locations were not visualized with the antibody used in this study, changes in the overall density of these receptors cannot be excluded. However, consistent with the present findings, a previous study found no difference in the density of axons that were immunoreactive for the serotonin transporter in the prefrontal cortex of subjects with schizophrenia, relative to matched comparison subjects with no psychiatric disorder (10).
Although the findings of this study were negative, they may be informative for the pathophysiology of schizophrenia. Specifically, studies in monkeys have revealed that adolescence is associated with marked changes in both pre- and postsynaptic markers of GABA neurotransmission at the pyramidal neuron axon initial segment (11), whereas markers of 5-HT neurotransmission at the axon initial segment are stable during this developmental epoch (12). In concert with the studies reviewed earlier, the current findings suggest that the maturation of GABA-mediated inhibition at the pyramidal neuron axon initial segment may be more relevant than 5-HT-mediated inhibition for the appearance of the clinical features of schizophrenia during late adolescence or early adulthood.
Received March 10, 2003; revision received Sept. 9, 2003; accepted Sept. 11, 2003. From the Departments of Psychiatry and Neuroscience, University of Pittsburgh; and the Departments of Biology and Psychiatry, New York University, New York. Address reprint requests to Dr. Lewis, Department of Psychiatry, University of Pittsburgh, 3811 O’Hara St., W1651 BST, Pittsburgh, PA 15213; [email protected] (e-mail). Supported by NIMH grants MH-43784, MH-45156, and MH-55250. The authors thank Mary Brady for assistance with the photomicrographs.
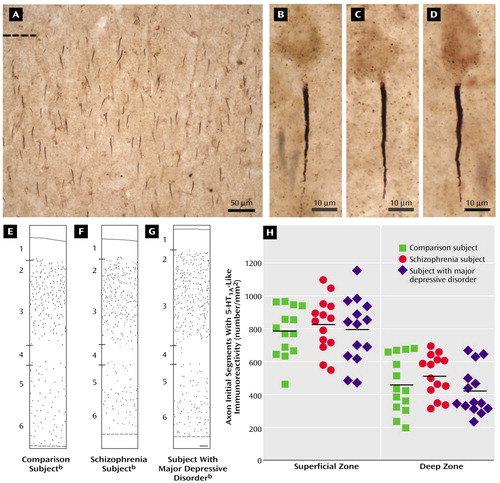
Figure 1. Density and Laminar Distribution of Axon Initial Segments With 5-HT1A-Like Immunoreactivity in Brodmann’s Area 46 of the Prefronal Cortex in Deceased Subjects With Schizophrenia, Subjects With Major Depressive Disorder, and Comparison Subjects With No Psychiatric Disordera
aPanel A shows a bright-field photomicrograph of axon initial segments labeled with an antibody against the 5-HT1A receptor in the superficial layers of Brodmann’s area 46 of the prefrontal cortex in a comparison subject with no psychiatric disorder. The dashed line indicates the border between layer 1 and layer 2 of the cortex. Panels B, C, and D show brightfield photomicrographs of labeled axon initial segments in a comparison subject with no psychiatric disorder (panel B), a subject with schizophrenia (panel C), and a subject with major depressive disorder (panel D). The segments appear as vertically oriented processes that are located below the unlabeled cell bodies of pyramidal neurons and that taper slightly from superficial to deep layers. Panels E, F, and G show Neurolucida (MicroBrightField, Inc., Williston, Vt.) drawings illustrating the laminar distribution of labeled axon initial segments in Brodmann’s area 46 of the prefrontal cortex in a comparison subject with no psychiatric disorder (panel E), a subject with schizophrenia (panel F), and a subject with major depressive disorder (panel G). Each drawing represents a 450-μm-wide traverse from the pial surface (solid line) to the white matter border (dashed line). Hash marks indicate the location of laminar boundaries. Panel H shows the mean densities of labeled axon initial segments in individual comparison subjects, subjects with schizophrenia, and subjects with major depressive disorder. The horizontal lines represent the mean value for each diagnostic group. No between-group differences were observed in either the superficial (F=0.38, df=2, 35, p=0.69) or deep (F=1.77, df=2, 35, p=0.19) zones of Brodmann’s area 46.
bThe scale bar (100 μm) shown in panel G applies to panels E, F, and G.
1. Pierri JN, Chaudry AS, Woo T-UW, Lewis DA: Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry 1999; 156:1709–1719Abstract, Google Scholar
2. Volk DW, Pierri JN, Fritschy J-M, Auh S, Sampson AR, Lewis DA: Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex 2002; 12:1063–1070Crossref, Medline, Google Scholar
3. DeFelipe J, Arellano JI, Gómez A, Azmitia EC, Muñoz A: Pyramidal cell axons show a local specialization for GABA and 5-HT inputs in monkey and human cerebral cortex. J Comp Neurol 2001; 433:148–155Crossref, Medline, Google Scholar
4. Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN: Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther 2001; 92:179–212Crossref, Medline, Google Scholar
5. Bantick RA, Deakin JFW, Grasby PM: The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics. J Psychopharmacol 2001; 15:37–46Crossref, Medline, Google Scholar
6. Tauscher J, Kapur S, Verhoeff NP, Hussey DF, Daskalakis ZJ, Tauscher-Wisniewski S, Wilson AA, Houle S, Kasper S, Zipursky RB: Brain serotonin 5-HT(1A) receptor binding in schizophrenia measured by positron emission tomography and [11C]WAY-100635. Arch Gen Psychiatry 2002; 59:514–520Crossref, Medline, Google Scholar
7. Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM: Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology 1996; 14:35–46Crossref, Medline, Google Scholar
8. Pucak ML, Levitt JB, Lund JS, Lewis DA: Patterns of intrinsic and associational circuitry in monkey prefrontal cortex. J Comp Neurol 1996; 376:614–630Crossref, Medline, Google Scholar
9. Maccaferri G, Roberts JDB, Szucs P, Cottingham CA, Somogyi P: Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol 2000; 524:91–116Crossref, Medline, Google Scholar
10. Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA: Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 1999; 156:1580–1589Link, Google Scholar
11. Cruz DA, Eggan SM, Lewis DA: Postnatal development of pre- and post-synaptic GABA markers at chandelier cell inputs to pyramidal neurons in monkey prefrontal cortex. J Comp Neurol 2003; 465:385–400Crossref, Medline, Google Scholar
12. Eggan SM, Cruz DA, Azmitia EC, Lewis DA: Postnatal development of 5-HT1A receptors at the axon initial segment of pyramidal neurons in monkey prefrontal cortex. Abstracts of the Society for Neuroscience 2002; 28:741.2Google Scholar



