Reduction of Behavioral Disturbances and Caregiver Distress by Galantamine in Patients With Alzheimer’s Disease
Abstract
OBJECTIVE: Alzheimer’s disease pathology includes both histologic changes and neurotransmitter deficits. The cholinergic deficit contributes to both cognitive and behavioral disturbances, and cholinesterase inhibitors may improve behavior in Alzheimer’s disease patients. This analysis was conducted to assess the impact of galantamine, a cholinesterase inhibitor with nicotinic-receptor-modulating properties, on the pattern and evolution of behavioral disturbances in patients with Alzheimer’s disease and on caregiver distress related to patients’ behavior. METHOD: Data from 978 patients with mild to moderate Alzheimer’s disease who were randomly assigned to placebo or galantamine (8, 16, or 24 mg/day) were analyzed. Behavioral changes were assessed with the Neuropsychiatric Inventory, and alterations in caregiver distress were measured by the Neuropsychiatric Inventory distress scale. Data collected at baseline and 12 and 21 weeks postbaseline were analyzed. RESULTS: Neuropsychiatric Inventory scores worsened with placebo, whereas patients treated with 16 or 24 mg/day of galantamine had no change in total Neurospcyhiatric Inventory scores. Treated patients, asymptomatic or symptomatic at baseline, had better Neuropsychiatric Inventory subscale scores than did patients receiving placebo. Behavioral improvement in patients symptomatic at baseline ranged from 29% to 48%. Changes were evident in patients receiving 16 or 24 mg/day of galantamine. High-dose galantamine was associated with a significant reduction in caregiver distress. CONCLUSIONS: Galantamine therapy was associated with reduced emergence of behavioral disturbances and improvement in existing behavioral problems in patients with mild to moderate Alzheimer’s disease, with a concomitant reduction in reported caregiver distress.
The pathology of Alzheimer’s disease includes both histologic changes and neurotransmitter deficits. The cholinergic deficit of this disorder contributes to both cognitive and behavioral disturbances. Galantamine is a tertiary alkaloid, originally derived from plants, that reversibly and competitively inhibits acetylcholinesterase and allosterically modulates nicotinic acetylcholine receptors (1, 2). In clinical trials involving patients with Alzheimer’s disease, galantamine was shown to improve or maintain global function, cognitive abilities, and activities of daily living better than placebo (3–5).
Cholinesterase inhibitors have been observed to have psychotropic effects, including effects on apathy, hallucinations, anxiety, depression, agitation, and delusions, in various clinical trials (6). In a double-blind, placebo-controlled, parallel-group trial of galantamine in Alzheimer’s disease (4), patients treated with 16 or 24 mg/day of the cholinesterase inhibitor galantamine had no overall change in behavior during the 21-week study, whereas patients taking placebo had significantly worsened scores on the Neuropsychiatric Inventory (7) at the end of the study. The current investigation was based on a hypothesis-driven secondary analysis of the behavioral data derived from the original study. We hypothesized that changes on the Neuropsychiatric Inventory would correlate with global ratings and with assessments of instrumental activities of daily living, but not with cognition. We posited a dissociation between cognition and behavioral responses on the basis of evidence that these disease manifestations are mediated by different brain regions. We further hypothesized that reductions in psychopathology would be associated with reduced behavior-related caregiver distress. We also sought to determine whether galantamine reduced existing psychopathology and/or reduced the emergence of new neuropsychiatric symptoms.
Method
Patient Selection
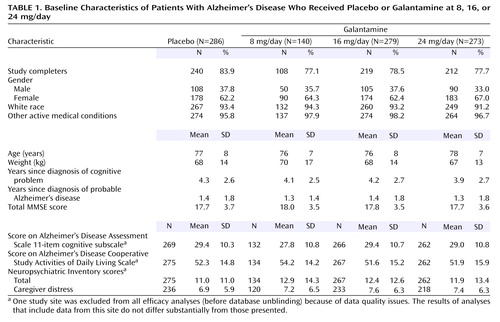
The patients included in the trial had a diagnosis of probable Alzheimer’s disease based on the criteria of the work group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (8). Patients had scores on the Mini-Mental State Examination (MMSE) (9) between 10 and 22 and scores of 18 or higher on the standard version of the Alzheimer’s Disease Assessment Scale cognitive subscale (10). Laboratory studies and neuroimaging were obtained to support the diagnosis of probable Alzheimer’s disease and exclude other potential causes of dementia, a practice consistent with the guidelines of the American Academy of Neurology (11).
Patients were excluded from the study if they had evidence of any other neurodegenerative disease or active cardiac, renal, pulmonary, metabolic, or endocrine disorder. Patients with a history of epilepsy or significant drug or alcohol abuse and those who had received an experimental agent in the preceding 60 days also were excluded, as were those with a history of major mental illness (schizophrenia, bipolar illness, or major depression) that was clinically evident within 5 years of the onset of the dementia.
Psychotropic medications were not a basis for exclusion from study participation. In the placebo group, 27% of the patients received antidepressants and 23% received other psychotropic medications (anxiolytics, hypnotics, and antipsychotics). In the three galantamine treatment arms, 26%–34% received antidepressants and 24%–27% received other psychotropic medications. Differences in medication type and dose among the groups were not significant. The use of hypnotics was prohibited for 48 hours before neuropsychological assessment.
A patient was considered eligible only if he or she had a responsible caregiver who, together with the patient, provided written informed consent in accordance with the Declaration of Helsinki and provided information on the patient’s behavior and his or her own response to the behavior in an interview using the Neuropsychiatric Inventory.
Study Design
The data used in the current analysis were derived from a multicenter, parallel-group, double-blind, placebo-controlled trial conducted in the United States (4). Following a 4-week single-blind, placebo lead-in period, the patients were randomly assigned to one of four treatment arms for 21 weeks (5 months): placebo, galantamine at a dose of 8 mg/day, galantamine at 16 mg/day, or galantamine at 24 mg/day. The patients in the 16-mg/day group initially received 8 mg/day for 4 weeks; they then received 16 mg/day for 17 weeks. Patients in the 24-mg/day group received 8 mg/day for 4 weeks, then 16 mg/day for weeks 5–8, and then 24 mg/day for the remaining 13 weeks. Galantamine and placebo were administered as identical single tablets taken orally twice daily. The patients were assigned to the placebo, 8-mg, 16-mg, and 24-mg groups in a ratio of 2:1:2:2. Data were available from administration of the Neuropsychiatric Inventory at baseline, week 12, and week 21 of the 21-week study.
Patients were not selected or assigned to treatment on the basis of information derived with the Neuropsychiatric Inventory. Inspection of the group assignments revealed that the random assignment produced acceptable proportions of patients with and without baseline symptoms according to the Neuropsychiatric Inventory. The scores were somewhat low compared to typical clinical populations (7), reflecting a relatively limited amount of psychopathology; this is common among patients participating in randomized clinical trials. Including patients receiving psychotropic medication in the trial also may have reduced baseline scores.
Outcome Measures
The outcomes of interest in the current study were the Neuropsychiatric Inventory total score, individual behavioral domain scores, and the behavior-related caregiver distress score. The Neuropsychiatric Inventory assesses 10 behavioral domains commonly encountered in patients with Alzheimer’s disease: delusions, hallucinations, agitation/aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability/lability, and aberrant motor behavior. The Neuropsychiatric Inventory has been demonstrated to be valid and to have good interrater and test-retest reliability (7).
When administering the Neuropsychiatric Inventory, a clinician interviews a caregiver familiar with the patient’s behavior using a scripted interview, and the caregiver rates each domain as present or absent. If behavioral disturbances are present, they are scored on a 1–4-point scale by the caregiver for frequency and on a 1–3-point scale for severity with anchored ratings. The score for each domain is a product of the ratings for frequency and severity (thus, the maximum possible score is 12 for each domain). A total Neuropsychiatric Inventory score is generated by summing the 10 domain scores (total possible score=120). The Neuropsychiatric Inventory includes an integrated measure of behavior-related caregiver distress (12). After the caregiver rates the frequency and the severity of each behavior, he or she is asked to score their distress as it relates to the patient’s behavior on an anchored 0–5-point rating scale. The total caregiver distress score is generated by adding the item-related distress scores (total possible score=50).
Other primary and secondary efficacy measures used during the trial included the Alzheimer’s Disease Assessment Scale cognitive subscale, the Clinician’s Interview-Based Impression of Change, the Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale, and measures of adverse events. The cognitive subscale of the Alzheimer’s Disease Assessment Scale is a standardized assessment tool for measuring cognitive function in Alzheimer’s disease; the score can range from 0 to 70, with a lower score meaning better cognitive function (10). The Clinician’s Interview-Based Impression of Change with caregiver input is used to evaluate global change in patient cognitive function, behavior, and instrumental activities of daily living on a scale of 1 to 7, with a score of 4 indicating no change (13). The Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale determines the ability to perform activities of daily living; scores range from 0 to 78, with a higher score indicating better functioning (14).
Statistical Analysis
The total score on the Neuropsychiatric Inventory, the Neuropsychiatric Inventory caregiver distress score, and the total score on the Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale were used as secondary outcome measures in the original study (4). The primary efficacy measures—change from baseline to week 21 in the scores on the Alzheimer’s Disease Assessment Scale cognitive subscale and the Clinician’s Interview-Based Impression of Change with caregiver input—were presented previously (4). The calculations of group size and statistical power were based on the primary hypotheses to demonstrate treatment effect, according to these primary efficacy measures. The analysis of the change in the total of the 10 Neuropsychiatric Inventory item scores from baseline to week 21 used the same methods as the analyses of scores on the Alzheimer’s Disease Assessment Scale cognitive subscale and the Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale.
Two types of comparisons were made among the three galantamine groups and one placebo group in this study: comparisons between each galantamine dose and placebo and comparisons among galantamine doses. A step-down closed testing procedure (15) was used for both types of comparisons. The step-down procedure was defined a priori in a sequence of hypotheses in hierarchical order. The first comparison was between 24 mg/day and placebo. If this was significant at the 0.05 level, then the next comparison was between 16 mg/day and placebo. Each comparison could be made at the 0.05 level because of the sequential closed testing procedures. No adjustment for the Alzheimer’s Disease Assessment Scale cognitive subscale and Clinician’s Interview-Based Impression of Change was needed because results for these tests were not primary measures in the current analyses.
If the primary analysis showed that both 24 mg/day and 16 mg/day were statistically significant from placebo, testing proceeded to compare the 8-mg/day dose with placebo at the 0.05 level. A test for increasing response with increasing dose was done by using a linear contrast to explore the dose-response relationships among these doses. A similar step-down testing procedure was applied in the comparison of each of the higher doses of galantamine with the 8-mg dose.
All randomly assigned patients who received at least one dose of study medication were included in the analysis of data on safety, demographic variables, and baseline characteristics. The efficacy data were analyzed on the basis of observed cases, traditional last observation carried forward, classical intent-to-treat, and retrieved dropouts. (Traditionally, the “observed case” data set refers to double-blind data that are observed at designated assessment times. “Last observation carried forward” refers to the last observation available for each subject in the double-blind period, excluding baseline. The “classical intent-to-treat” data set comprises the last observation available for each subject during the study regardless of the time at which it was recorded. “Retrieved dropout data” are data retrieved and assessed at or close to the scheduled time point for patients who did not complete the study.) Patients with no postbaseline efficacy measurements were included in the classical intent-to-treat analysis set (if a datum was missing at a postbaseline time point, then the missing value was imputed from the baseline value) but were excluded from the analysis of the last observation carried forward (i.e., baseline data were not carried forward to the postbaseline time points in this set). The primary analysis was based on observed cases.
For the continuous variables (e.g., changes from baseline in scores on the Alzheimer’s Disease Assessment Scale cognitive subscale, total Neuropsychiatric Inventory, and total Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale), a two-way analysis of variance model with treatment and site as factors was used to compare the treatment groups for the change from baseline. The treatment-by-site interaction was tested and removed from the model when it was found not significant at the 10% level. The linear contrasts in the least-square means of the treatment effect were used to perform the between-group comparisons. The baseline value for each measure was included in an analysis of covariance model with treatment and site as factors and the baseline value as covariate. The baseline value was excluded from the analysis of each score in the final model, as the conclusions remained the same with or without it as a covariate in the model. A longitudinal analysis was performed for the continuous variables by applying a mixed-effects model (16)—with treatment, site, and treatment-by-week as factors and with an assumption of unstructured covariance—to evaluate the effect of treatment with galantamine over time. The score on the Clinician’s Interview-Based Impression of Change with caregiver input was analyzed by using the van Elteren test (17), i.e., the Cochran-Mantel-Haenszel test statistics based on modified ridit scores (derived from rank scores).
Results
A total of 1,178 patients were screened, and 978 were randomly assigned to medication or placebo, 80% of whom completed the trial. Table 1 presents the clinical characteristics of the randomly assigned patients. Patients had manifested symptoms for approximately 4 years and had been diagnosed with Alzheimer’s disease for approximately 1.5 years. The distribution of the baseline data was similar across treatment groups.
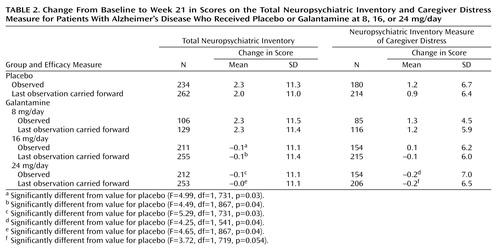
Table 2 summarizes the results from the analyses of the change from baseline to week 21 in the total score on the Neuropsychiatric Inventory and the score on the measure of caregiver distress. The analyses of observed cases revealed differences in favor of galantamine for the 16-mg/day and 24-mg/day groups.
After 21 weeks, the total score on the Neuropsychiatric Inventory was reduced by 0.1 point in the 16- and 24-mg/day galantamine groups and increased by 2.3 points in the placebo and 8-mg/day galantamine groups. The differences between galantamine and placebo in favor of galantamine were statistically significant for the 16-mg/day group (difference in least-square means=–2.5 points, 95% CI=–4.6 to –0.3) and the 24-mg/day group (difference in least-square means=–2.5 points, 95% CI=–4.7 to –0.4).
Analysis of the 21-week scores on subscales of the Neuropsychiatric Inventory revealed significant differences in favor of galantamine in aberrant motor behavior for the 16-mg/day group (difference from placebo in least-square means=–0.7, 95% CI=–1.2 to –0.2) and the 24-mg/day group (difference from placebo in least-square means=–0.7, 95% CI=–1.2 to –0.2). There were significantly greater reductions in anxiety, compared with placebo, for the 16-mg/day group (difference in least-square means=–0.6, 95% CI=–1.1 to –0.1) and the 24-mg/day group (difference in least-square means=–0.5, 95% CI=–1.1 to –0.0). Drug-placebo differences approached significance (difference in least-square means=–0.3, 95% CI=–0.5 to –0.0) for the hallucinations subscale score for the group taking 24 mg/day of galantamine. Behavior-related caregiver distress was significantly reduced in the caregivers of patients receiving 24 mg/day (difference in least-square means=–1.5, 95% CI=–2.9 to –0.1) compared with placebo.
This trial included two groups of patients with respect to baseline Neuropsychiatric Inventory scores: one without specific behavioral symptoms and one including patients who were symptomatic in one or more behavioral domains at baseline. The patients who were asymptomatic at study entry (with a score of zero on the Neuropsychiatric Inventory individual items) could have become symptomatic during the course of the illness or could have remained without symptoms. If the latter occurred with greater frequency in the treatment group than in the placebo group, the active treatment may have suppressed the emergence of behavioral disorders during the trial. The patients who were symptomatic (nonzero scores on the Neuropsychiatric Inventory items) at baseline could have improved, worsened, or shown no change during the trial. Treated patients who improved more than did those in the placebo group or who worsened less than did those in the placebo group may have derived psychotropic benefit from treatment. Our analyses took these possibilities into account.
An observed-case analysis of patients who were without specific behavioral symptoms at baseline revealed significantly less emergence of new behaviors by week 21 in the patients receiving galantamine on the following subscales: aberrant motor behavior (8-mg and 24-mg/day groups), apathy (16-mg/day group), and disinhibition (16-mg/day group). Patients without these symptoms at baseline and assigned to placebo developed these symptoms more frequently than did those assigned to galantamine. There was minimally greater emergence of agitation in those receiving galantamine. The magnitude of the drug-placebo differences was small, representing 0.3–0.8 points on the Neuropsychiatric Inventory.
Among the patients who were symptomatic at baseline, the score on the aberrant motor behavior scale showed greater reduction with galantamine than with placebo for the 16-mg/day dose (difference in least-square means=–1.4, 95% CI=–2.4 to –0.3) and the 24-mg/day dose (least-square mean difference=–0.9, 95% CI=–2.0 to 0.1). Patients with agitation/aggression had greater reductions in scores with galantamine at 16-mg/day (difference in least-square means=–1.5, 95% CI=–2.4 to –0.5) or 24-mg/day (difference in least-square means=–1.0, 95% CI=–2.0 to –0.1) than with placebo. The anxiety scale scores were also reduced more in the 16-mg/day group (difference in least-square means=–1.2, 95% CI=–2.2 to –0.2) and the 24-mg/day group (difference in least-square means=–1.1, 95% CI=–2.1 to –0.1) than in the placebo group. These reductions were clinically meaningful, with reductions from baseline of 38% and 29% (at 16 mg/day and 24 mg/day, respectively) in scores for aberrant motor behavior, 48% and 32% (16 and 24 mg/day) in agitation scores, and 41% and 29% (16 and 24 mg/day) in anxiety scores. Analysis of the 12-week data showed similar patterns in the groups that were symptomatic and asymptomatic at baseline, but only the smaller increases in anxiety in patients taking galantamine, compared to those taking placebo, among the initially asymptomatic group and the smaller increases in agitation in the symptomatic group reached statistical significance.
The change in the Neuropsychiatric Inventory total score was significantly but weakly associated with the score on the Clinician’s Interview-Based Impression of Change at week 21 in the placebo group (r=0.19, 95% CI=0.06 to 0.31, N=234) and in the 24-mg/day treatment group (r=0.25, 95% CI=0.12 to 0.37, N=212). Reduction in the total Neuropsychiatric Inventory score, i.e., improved behavior, also was associated with improvement in the score for activities of daily living in the placebo group (r=–0.13, 95% CI=–0.26 to –0.01, N=234) and in the 24-mg/day group (r=–0.14, 95% CI=–0.27 to –0.00, N=212). There was no significant association between change in the score on the Alzheimer’s Disease Assessment Scale cognitive subscale and change in the Neuropsychiatric Inventory score.
The change in total Neuropsychiatric Inventory score during the 21 weeks was associated significantly with the change in the distress score for all treatment groups (r≥0.68, 95% CI from ≥0.59 to ≥0.76).
A longitudinal analysis of the total Neuropsychiatric Inventory score was performed to assess the effect of galantamine treatment as measured by the total score over time. There was a significant interaction of time (week) with treatment (F=4.89, df=4, 1586, p=0.001), indicating that the rates of changes in the total score were significantly different among the treatment groups. Both 16 mg/day (t=2.83, df=1,586, p=0.005) and 24 mg/day (t=2.55, df=1,586, p=0.02) of galantamine were significantly more effective than was placebo.
Discussion
This hypothesis-driven analysis of changes in neuropsychiatric symptoms was performed to further explore the effects of galantamine on behavior. The results of post hoc analyses of this type must be interpreted cautiously, as the patients were not recruited or randomly assigned on the basis of behavioral symptoms. Emergence of aberrant motor behavior, apathy, and disinhibition was somewhat less in galantamine-treated patients who were without these symptoms at baseline. In patients who exhibited behavioral abnormalities at the time of study entry, there were robust reductions from baseline (29%–48%) in aberrant motor behavior, agitation, and anxiety. In addition, the treatment-related benefit to caregivers on the behavior-related caregiver distress measure of the Neuropsychiatric Inventory is consonant with a psychotropic effect of galantamine.
Effects of cholinesterase inhibitors on behavior have been observed in open-label trials of tacrine (18) and rivastigmine (19) and in double-blind, placebo-controlled trials of metrifonate (20) and donepezil (21). Tacrine diminished apathy, anxiety, disinhibition, and aberrant motor behavior (18). Rivastigmine reduced mood disorders and hallucinations in a 2-year open-label extension of a double-blind clinical trial (19). Metrifonate reduced the score on the Neuropsychiatric Inventory subscale for visual hallucinations, as well as the total Neuropsychiatric Inventory score (20). Donepezil therapy was associated with a reduced total Neuropsychiatric Inventory score and improved scores on the anxiety, depression, and apathy subscales in patients with moderate-to-severe Alzheimer’s disease (21). A community survey of patients receiving donepezil revealed that they were less likely to receive prescriptions for psychotropic agents than were patients not receiving donepezil (22). Thus, psychotropic effects are commonly reported across studies of cholinesterase inhibitors. The behavioral response profiles, however, differ somewhat from study to study. These differing symptom responses may reflect differences in the populations included in the trials, differences in the pharmacologic properties of the agents studied, or differences in the behavioral measures employed.
To our knowledge, no previous study of an approved cholinesterase inhibitor has shown a reduction in the emergence of new neuropsychiatric symptoms in patients who were asymptomatic at baseline. In the current study, there were modest but significant reductions in the emergence of aberrant motor behavior, apathy, and disinhibition. Similar reductions were observed in clinical trials of metrifonate (23), a cholinesterase inhibitor whose further development was discontinued. Reduced emergence of new behavioral disturbances may be a benefit of long-term cholinesterase inhibitor therapy.
The current report also appears to be the first to identify a beneficial impact of cholinesterase inhibitor therapy on behavior-related caregiver distress. The difference in Neuropsychiatric Inventory caregiver distress scores between patients receiving active drug and those receiving placebo was approximately 15%, indicative of lower caregiver distress for individuals given active therapy. Specific areas of patient improvement include depression/dysphoria, disinhibition, hallucinations, and irritability/lability. A previous study with the atypical antipsychotic olanzapine showed reduced reports of occupational disruption by nursing home caregivers of patients who exhibited reduced delusions, hallucinations, and agitation following treatment (24). Reduction in caregiver distress is an important outcome in treatment of patients with Alzheimer’s disease, because caregiver stress and burden lead to caregiver illness and may precipitate institutionalization of patients with Alzheimer’s disease (25, 26). Although reduced caregiver distress could be attributed to improved cognition or function, the Neuropsychiatric Inventory assesses distress specifically linked to behaviors, and the reductions observed are likely attributable to the improved behavior.
Alterations in function as measured by changes in the Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale correlated significantly with Neuropsychiatric Inventory scores. Activities of daily living require both instrumental cognitive functions, such as memory, and cognitive functions mediated by the frontal lobes. Behavioral abnormalities also have been related to frontal lobe dysfunction (27); frontal mediation of both activities of daily living and emotion and behavior may explain the correlation between these measures (28). Performance on the Alzheimer’s Disease Assessment Scale cognitive subscale depends primarily on posterior hemispheric regions that support language, memory, and visuospatial skills. There are no measures of executive function on the standard version of the Alzheimer’s Disease Assessment Scale cognitive subscale reported here. The absence of correlations between cognitive and behavioral changes following treatment with galantamine indicates that these two domains are independent response dimensions.
There are multiple mechanisms by which galantamine may exert psychotropic effects. First, the acetylcholine deficiency of Alzheimer’s disease is marked in the limbic system (29), and enhancement of limbic function with a cholinesterase inhibitor may ameliorate behavioral alterations. Second, the allosteric nicotinic modulation exerted by galantamine increases arousal and may decrease aberrant motor behavior and agitation, similar to the effects of psychostimulants in improving symptoms of hyperactivity in children with attention deficit disorders (30). Third, improved function of nicotinic thalamofrontal projections may reduce agitation, disinhibition, and apathy, which appear to be frontally mediated (31, 32). Fourth, galantamine has secondary effects on other transmitters (33, 34), and these subsequent events may be partially responsible for the psychotropic effects of the agent. Finally, improvements in global functioning, activities of daily living, and cognitive abilities may have secondary behavioral consequences.
In summary, patients treated with galantamine had better total Neuropsychiatric Inventory scores as well as less aberrant motor behavior, agitation, anxiety, apathy, and disinhibition than did patients receiving placebo. There was an attendant reduction in behavior-related caregiver distress. Improved behavior contributed to the global benefit of galantamine and was correlated with changes in scores on the Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale. This analysis suggests that galantamine has psychotropic properties in addition to enhancing function and cognition.
 |
 |
Received Nov. 28, 2001; revision received July 9, 2003; accepted July 24, 2003. From the Department of Neurology and the Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine at UCLA; the Department of Psychiatry and Behavioral Sciences, Keck School of Medicine, University of Southern California, Los Angeles; the Department of Psychiatry, University of Rochester School of Medicine, Rochester, N.Y.; the Department of CNS, Janssen Pharmaceutica, Titusville, N.J.; and Janssen Research Foundation, Titusville, N.J. Address reprint requests to Dr. Cummings, Reed Neurological Research Center, David Geffen School of Medicine at UCLA, 710 Westwood Plaza, Los Angeles, CA 90095-1769; [email protected] (e-mail). Dr. Cummings’s work was supported by a National Institute on Aging Alzheimer’s Disease Research Center grant (AG-16570), an Alzheimer’s Disease Research Center of California grant, and the Sidell-Kagan Foundation.
1. Maelicke A, Schrattenholz A, Samochocki M, Radina M, Albuquerque EX: Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer’s disease. Behav Brain Res 2000; 113:199–206Crossref, Medline, Google Scholar
2. Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A: Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther 1997; 280:1117–1136Medline, Google Scholar
3. Raskind MA, Peskind ER, Wessel T, Yuan W: Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. Neurology 2000; 54:2261–2268Crossref, Medline, Google Scholar
4. Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C: A 5-month, randomized, placebo-controlled trial of galantamine in AD. Neurology 2000; 54:2269–2276Crossref, Medline, Google Scholar
5. Wilcock G, Wilkinson D: Galanthamine hydrobromide: interim results of a group comparative, placebo-controlled study of efficacy and safety in patients with a diagnosis of senile dementia of the Alzheimer type, in Alzheimer’s Disease: Biology, Diagnosis and Therapeutics. Edited by Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski HM. New York, John Wiley & Sons, 1997, pp 661–664Google Scholar
6. Cummings JL: Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry 2000; 157:4–15Link, Google Scholar
7. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
8. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
9. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
10. Rosen WG, Mohs RC, Davis KL: A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984; 141:1356–1364Link, Google Scholar
11. Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC: Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143–1153Crossref, Medline, Google Scholar
12. Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, MacMillan A, Ketchel P, DeKosky ST: Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 1998; 46:210–215Crossref, Medline, Google Scholar
13. Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH: Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11(suppl 2):S22-S32Google Scholar
14. Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S: An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11(suppl 2):S33-S39Google Scholar
15. Marcus R, Peritz E, Gabriel KR: On closed testing procedures with special reference to ordered analysis of variance. Biometrika 1976; 63:655–660Crossref, Google Scholar
16. Laird NM, Ware JH: Random-effects models for longitudinal data. Biometrics 1982; 38:963–974Crossref, Medline, Google Scholar
17. van Elteren PH: On the combination of independent two sample tests of Wilcoxon. Bulletin de L’Institut International De Statistique (Bruxelles) 1960, pp 351–361Google Scholar
18. Kaufer DI, Cummings JL, Christine D: Effect of tacrine on behavioral symptoms in Alzheimer’s disease: an open-label study. J Geriatr Psychiatry Neurol 1996; 9:1–6Crossref, Medline, Google Scholar
19. Rosler M, Retz W, Retz-Junginger P, Dennler HJ: Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer’s disease. Behav Neurol 1998; 11:211–216Crossref, Medline, Google Scholar
20. Morris JC, Cyrus PA, Orazem J, Mas J, Bieber F, Ruzicka BB, Gulanski B: Metrifonate benefits cognitive, behavioral, and global function in patients with Alzheimer’s disease. Neurology 1998; 50:1222–1230Crossref, Medline, Google Scholar
21. Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E: A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology 2001; 57:613–620Crossref, Medline, Google Scholar
22. Small GW, Donohue JA, Brooks RL: An economic evaluation of donepezil in the treatment of Alzheimer’s disease. Clin Ther 1998; 20:838–850Crossref, Medline, Google Scholar
23. Cummings JL, Nadel A, Masterman D, Cyrus PA: Efficacy of metrifonate in improving the psychiatric and behavioral disturbances of patients with Alzheimer’s disease. J Geriatr Psychiatry Neurol 2001; 14:101–108Crossref, Medline, Google Scholar
24. Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, Tamura RN, Mitan SJ, Kadam DL, Sanger TM, Feldman PD, Tollefson GD, Breier A: Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2000; 57:968–976Crossref, Medline, Google Scholar
25. Buckwalter KC: Overview of psychological factors contributing to stress of family caregivers, in Alzheimer’s Disease: Causes, Diagnosis, Treatment, and Care. Edited by Khachaturian ZS, Radebaugh TS. New York, CRC Press, 1996, pp 305–312Google Scholar
26. Maheu S, Cohen CA: Support of families, in Clinical Diagnosis and Management of Alzheimer’s Disease. Edited by Gauthier S. London, Martin Dunitz, 1999, pp 307–318Google Scholar
27. Cummings JL: Cognitive and behavioral heterogeneity in Alzheimer’s disease: seeking the neurological basis. Neurobiol Aging 2000; 21:845–861Crossref, Medline, Google Scholar
28. Tekin S, Fairbanks LA, O’Connor S, Rosenberg S, Cummings JL: Activities of daily living in Alzheimer’s disease: neuropsychiatric, cognitive and medical illness influences. Am J Geriatr Psychiatry 2001; 9:81–86Crossref, Medline, Google Scholar
29. Guela C, Mesulam M-M: Cholinergic systems and related neuropathological predilection patterns in Alzheimer disease, in Alzheimer Disease. Edited by Terry RD, Katzman R, Bick KL. New York, Raven Press, 1994, pp 263–291Google Scholar
30. Rezvani AH, Levin ED: Cognitive effects of nicotine. Biol Psychiatry 2001; 49:258–267Crossref, Medline, Google Scholar
31. Arneric SP: Neurobiology and clinical pathophysiology of neuronal nicotinic acetylcholine receptors, in Nicotine in Psychiatry: Psychopathology and Emerging Therapeutics. Edited by Piasecki M, Newhouse PA. Washington, DC, American Psychiatric Press, 2000, pp 3–35Google Scholar
32. Craig AH, Cummings JL, Fairbanks L, Itti L, Miller BL, Li J, Mena I: Cerebral blood flow correlates of apathy in Alzheimer’s disease. Arch Neurol 1996; 53:1116–1120Crossref, Medline, Google Scholar
33. Dani JA: Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry 2001; 49:166–174Crossref, Medline, Google Scholar
34. Kaiser S, Wonnacott S: Nicotinic receptor modulation of neurotransmitter release, in Neuronal Nicotinic Receptors: Pharmacology and Therapeutic Opportunities. Edited by Arneric SP, Brioni JD. New York, Wiley-Liss, 1998, pp 141–149Google Scholar



