Hippocampus and Amygdala Volumes in Parents of Children With Autistic Disorder
Abstract
OBJECTIVE: Structural and functional abnormalities in the medial temporal lobe, particularly the hippocampus and amygdala, have been described in people with autism. The authors hypothesized that parents of children with a diagnosis of autistic disorder would show similar changes in these structures. METHOD: Magnetic resonance imaging scans were performed in 17 biological parents of children with a diagnosis of DSM-IV autistic disorder. The scans were compared with scans from 15 adults with autistic disorder and 17 age-matched comparison subjects with no personal or familial history of autism. The volumes of the hippocampus, amygdala, and total brain were measured in all participants. RESULTS: The volume of the left hippocampus was larger in both the parents of children with autistic disorder and the adults with autistic disorder, relative to the comparison subjects. The hippocampus was significantly larger in the adults with autistic disorder than in the parents of children with autistic disorder. The left amygdala was smaller in the adults with autistic disorder, relative to the other two groups. No differences in total brain volume were observed between the three groups. CONCLUSIONS: The finding of larger hippocampal volume in autism is suggestive of abnormal early neurodevelopmental processes but is partly consistent with only one prior study and contradicts the findings of several others. The finding of larger hippocampal volume for the parental group suggests a potential genetic basis for hippocampal abnormalities in autism.
The neuroanatomical pathology of autism is still poorly understood. Changes in the cerebellum, hippocampus, amygdala, basal ganglia, cerebral ventricles, and planum temporale have all been described (1–4), although some of the changes have not been replicated in follow-up studies. Medial temporal lobe structures such as the hippocampus and amygdala have been of particular interest because these limbic structures have been proposed to underlie key behavioral dysfunctions in autism (5). Bauman and Kemper (1) first reported evidence of higher cell packing density and smaller neuronal size in the hippocampus, amyg-dala, and entorhinal cortex and in several other structures in a single case autopsy study. These findings were subsequently replicated in a larger sample (6).
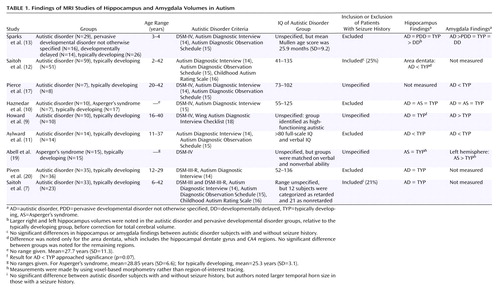
Noninvasive magnetic resonance imaging (MRI) studies have also been performed on the hippocampus and amygdala. The results for both structures are equivocal. Several studies of hippocampal volume have failed to find differences in children and adults with autism (7–10). There have been reports of smaller hippocampal volume (11, 12) as well as larger hippocampal volume (13) in autism, although the latter finding was true only before statistical correction for total brain volume. As with the hippocampus, some studies of the amygdala have had negative findings (10) and others have reported smaller amygdala volume (11) and larger amygdala volume (9, 13) in autism. Table 1 summarizes the published findings on hippocampus and amygdala volumes in autism.
To our knowledge, no studies of the medial temporal lobe in relatives of individuals with autism have been reported. Studies of unaffected relatives can yield information on which anatomical components of the disorder may be heritable. This information in turn may help resolve some ambiguity surrounding the direction (smaller or larger) of the deficit, if any. Studies of unaffected relatives also largely avoid confounding issues such as intellectual dysfunction, disorder history, and, in the case of parents, child and adolescent development.
For this study, we examined hippocampus and amygdala volumes in clinically unaffected parents of children with autistic disorder, adults with autistic disorder, and adults with no personal or familial history of autism. The current study was an attempt to establish the familiality of differences in medial temporal lobe structures that have been previously implicated in autistic disorder. We reasoned that a volumetric difference in autism would be most pronounced in the affected adults and somewhat less so in the unaffected parents of children with autism, relative to the comparison subjects, given the high genetic contribution to the disorder suggested by twin studies (21). This approach is similar to that of MRI studies of unaffected first-degree relatives of schizophrenia probands (e.g., references 22, 23), which have suggested that smaller hippocampal volume may be a genetically mediated neurobiological risk factor for schizophrenia (24). Given the ambiguity of the prior work on medial temporal lobe structures in individuals with autism, we could not specify a priori in what direction (smaller or larger) the differences, if seen, would appear.
Method
Subjects
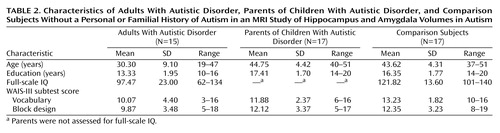
Seventeen biological parents of children with a diagnosis of autistic disorder were recruited to participate in this project (nine women). Their children met the clinical criteria for DSM-IV autistic disorder, as well as the criteria for autism of both the Autism Diagnostic Interview (14) and the Autism Diagnostic Observation Schedule (15). Each of the participating parents had only one child with a diagnosis of autistic disorder. Fifteen adult participants with DSM-IV autistic disorder were recruited (two women). They met the same criteria for autism described for the probands. Seventeen adult participants with no history of neurological or psychiatric disorders were recruited from the Denver metropolitan region to serve as comparison subjects. The comparison subjects were matched for chronological age and gender to the parents of children with autistic disorder (nine women). The comparison subjects and the parents of children with autistic disorder were screened with the Structured Clinical Interview for DSM-IV (SCID) Screen Patient Questionnaire—Extended (25), and subjects whose responses needed further clarification were administered that portion of the full SCID (26) still in question. Participants in the comparison group reported no personal history of neurological or axis I psychiatric illness and met the Research Diagnostic Criteria (27) for never mentally ill. In addition, comparison subjects had no reported family history of neurological or psychiatric illness. Two women among the parents of children with autistic disorder met the criteria for major depressive disorder and were taking selective serotonin reuptake inhibitor medication (citalopram and paroxetine) at the time of the study. Of the 15 adults with autistic disorder, only one had received a diagnosis of seizure disorder and was taking medication at the time of the study.
The comparison subjects and the adults with autistic disorder were administered a full WAIS-R (28) or WAIS-III (29) (Table 2). The parents of children with autistic disorder were administered only the vocabulary and block design subtests of the WAIS-III, so direct comparisons between all three groups are available only for those two measures from the Wechsler scales. Demographic and cognitive data for the participants are shown in Table 2. All subjects provided written informed consent to participate in the study.
MRI
T1-weighted (TR=40 msec, TE=5 msec, 40° flip angle) images were acquired on a 1.5-T system (G.E. Medical Systems, Inc., Milwaukee). A total of 124 contiguous, 1.7-mm thick coronal slices with a 192×256 (reconstructed to 2562) matrix in a 240-mm field of view resulted in voxel dimensions of 0.9375 mm by 0.9375 mm by 1.7 mm. Before the scan, the coronal slice axis was aligned perpendicular to a line connecting the anterior tip of the genu of the corpus callosum with the posterior tip of the splenium of the corpus callosum. Participants were not sedated for the scans.
Definition of MRI Regions of Interest
Custom software based on Interactive Data Language 5.3 (Research Systems, Inc., Boulder, Colo.) was used for MRI analyses. The software allows viewing and referencing of regions of interest in the coronal, axial, and sagittal planes simultaneously, although all segmentation was performed in the coronal plane. Ratings of structural measures were made blind to subject diagnosis. Total brain volume was measured by a semiautomated procedure in which the gray matter-CSF interface was automatically traced by a contour based on a pixel threshold determined by an experienced rater. The method for defining the amygdala and hippocampus has been previously described by Watson et al. (30) and was supplemented by the descriptions of Honeycutt et al. (31) with respect to the lateral and inferior boundaries of the hippocampus.
Although a single rater determined the volume of all structures, we determined both intrarater and interrater reliability for the total brain, hippocampus, and amygdala. Ten of each structure were randomly selected to be manually retraced. Intraclass correlation coefficients (ICCs) were calculated between the original and second segmentations and between one rater and a second rater. The intrarater ICCs were 0.99 for the total brain, 0.96 for the hippocampus, and 0.97 for the amygdala. The interrater ICCs were 0.97 for the total brain, 0.96 for the hippocampus, and 0.95 for the amygdala. We also computed the percentage of overlapping voxels within and between raters to account for the spatial relationships between ratings, which correlation coefficients do not address. The overlap was determined as the intersection of ratings 1 and 2 divided by the union of ratings 1 and 2. For the total brain, the mean intrarater overlap was 0.97 (SD=0.01) and the mean interrater overlap was 0.94 (SD=0.03). The mean intrarater overlap was 0.79 (SD=0.06) for the hippocampus and 0.82 (SD=0.04) for the amygdala. The mean interrater overlap was 0.74 (SD=0.05) for the hippocampus and 0.80 (SD=0.07) for the amygdala.
Data Analysis
All data analyses were conducted by using Statistica 5.3 (Statsoft, Tulsa, Okla.). Null hypothesis significance tests were two-tailed and used an alpha of 0.05. Fisher’s least significant difference post hoc tests were used to explore differences in factors with more than one degree of freedom. To detect overall differences between means on the demographic variables, separate one-way analysis of variance designs were employed with group as the single factor. Pearson product-moment correlation coefficients were calculated to examine relationships between demographic variables (age, education level, WAIS-III full-scale IQ, performance on the WAIS-III vocabulary and block design subtests) and the morphometric variables. Total brain volume was analyzed with a one-way analysis of covariance (ANCOVA) with group as the single factor and gender as a covariate. The left and right hippocampal volumes were separately analyzed with one-way ANCOVA designs, with group as the single factor and age, gender, and total brain volume as the covariates. The left and right amygdala volumes were also separately analyzed with one-way ANCOVA designs, with group as the single factor and total brain volume and gender as covariates.
Results
There was an overall age difference between the groups (F=26.25, df=2, 46, p<0.0001). This difference was entirely due to the inclusion of the adults with autistic disorder, who as a group were significantly younger than both the parents of children with autistic disorder (p<0.0001, Fisher’s least significant difference test) and the comparison group (p<0.0001, Fisher’s least significant difference test). The age-matched parents of children with autistic disorder and the comparison subjects did not differ in age (p=0.60, Fisher’s least significant difference test), as expected. The education level of the participants was also different between groups (F=21.66, df=2, 46, p<0.0001) and was significantly lower for the adults with autistic disorder than for the other two groups (p<0.0001 and p<0.0001, respectively, Fisher’s least significant difference test) but did not differ between the comparison subjects and the parents of children with autistic disorder (p=0.93, Fisher’s least significant difference test). The WAIS-III vocabulary scaled scores also differed between groups (F=4.46, df=2, 46, p<0.02). For the vocabulary subtest, the adults with autistic disorder and the comparison subjects differed (p<0.005, Fisher’s least significant difference test); the difference between the adults with autistic disorder and the parents of children with autistic disorder did not reach significance (p<0.10, Fisher’s least significant difference test). The parents of children with autistic disorder and the comparison subjects did not differ in vocabulary scores (p=0.19, Fisher’s least significant difference test). For the WAIS-III block design subtest, the groups did not differ (F=2.61, df=2, 46, p>0.05). For full-scale IQ, the adults with autistic disorder differed significantly from the comparison group (t=3.70, df=30, p<0.001). Group means and standard deviations for these measures are provided in Table 2.
The correlation analyses of the relationships between the demographic variables (age, education level, WAIS-III full-scale IQ, performance on the WAIS-III vocabulary and WAIS-III block design subtests) and the morphometric variables used a Bonferroni corrected alpha of p=0.002. Given that correction, the only significant correlations were between age and left and right hippocampal volumes (N=49, r=–0.51 and r=–0.46, respectively). Total brain volume correlated only with the other four volumetric measures: left hippocampus volume (r=0.55), right hippocampus volume (r=0.51), left amygdala volume (r=0.54), and right amygdala volume (r=0.47). Therefore, age and total brain volume were both used as covariates in analyses involving the hippocampus, and total brain volume was used as a covariate in analyses involving the amygdala.
Gender was also examined in relation to MRI volume measures. Left hippocampal volume was significantly larger in men (mean=4.71 ml, SD=0.43) than in women (mean=4.07 ml, SD=0.33) (t=5.62, df=47, p<0.0001). Right hippocampal volume was also larger in men (mean=4.73 ml, SD=0.47) than in women (mean=4.10 ml, SD=0.33) (t=5.26, df=47, p<0.0001). Both the left amygdala (men: mean=3.71 ml, SD=0.34; women: mean=3.34 ml, SD=0.34) and the right amygdala (men: mean=3.75 ml, SD=0.35; women: mean=3.38 ml, SD=0.30) were significantly larger in the men than the women (t=3.76, df=47, p<0.001, and t=3.92, df=47, p<0.001, respectively). Finally, total brain volumes differed significantly between men (mean=1302.70 ml, SD=113.03) and women (mean=1164.21 ml, SD=113.51) (t=4.21, df=47, p<0.001). Gender was therefore used as a covariate in all group comparisons of brain volumes.
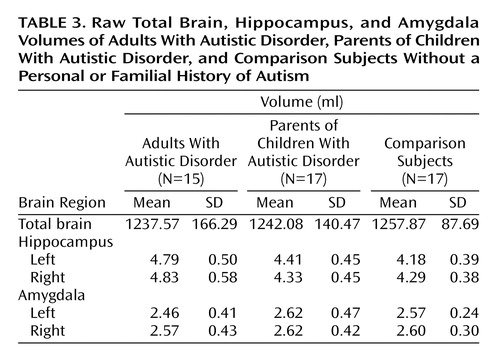
Although the mean total brain volumes (analyzed with a one-way ANCOVA with group as the single factor and gender as a covariate) for the adults with autistic disorder and the parents of children with autistic disorder (mean=1237.57 ml, SD=166.29, and mean=1242.08 ml, SD=140.46, respectively) were slightly smaller than the total mean brain volume for the comparison subjects (mean=1257.87 ml, SD=87.69), the difference did not reach statistical significance (F=2.34, df=2, 45, p=0.11).
In the separate analyses of left and right hippocampal volumes, the volume of the left hippocampus differed between groups (F=4.63, df=2, 43, p<0.02). Post hoc analyses revealed that the left hippocampus was significantly larger in the adults with autistic disorder than in both the parents of children with autistic disorder (p=0.002, Fisher’s least significant difference test) and the comparison group (p<0.0001, Fisher’s least significant difference test). The left hippocampus of the parents of children with autistic disorder was also significantly larger than that of the comparison subjects (p<0.05, Fisher’s least significant difference test). The volume of the right hippocampus did not differ significantly between groups (F=2.53, df=2, 43, p=0.09). The group means and standard deviations for the raw hippocampal volumes are provided in Table 3.
In the separate analyses of the left and right amygdala volumes, the volume of the left amygdala differed between groups (F=3.42, df=2, 44, p<0.05). Post hoc analyses revealed no groupwise differences, which most likely indicated that adjustment for the covariates of total brain volume and gender was necessary to achieve significance in the overall analysis. Indeed, reanalysis of the results without gender as a covariate yielded no significant difference between groups (F=0.95, df=2, 45, p>0.05). No significant differences were observed between groups for the right amygdala (F=1.13, df=2, 44, p>0.05), with or without the covariates included in the model. The group means and standard deviations for the raw amygdala volumes are provided in Table 3.
Discussion
We found larger left hippocampal volume in biological parents of children with autism, relative to age-matched healthy comparison subjects. Adults with autistic disorder were also included in the study to establish the directionality of differences in hippocampal volume that would suggest a genetic component if the difference also appeared in the parent group. The adults with autistic disorder showed larger left hippocampal volume, relative to both the comparison group and the parent group. Larger hippocampal volumes have been reported in other neurodevelopmental disorders such as fragile X syndrome (32, 33). This finding is provocative, in that fragile X syndrome is one of the few known genetic etiologies for autism. As many as 15% of children with fragile X syndrome, caused by a CGG trinucleotide expansion of the FMR1 gene on the X chromosome, also meet the diagnostic criteria for autistic disorder (34). Reiss and colleagues (32, 33) have also found age-dependent larger hippocampal volumes in subjects with fragile X syndrome, relative to age-matched comparison subjects. These findings may suggest a dysfunction in developmentally appropriate synaptic pruning, which has been described in fragile X syndrome (35, 36). At this point, however, speculation about the mechanisms involved in larger hippocampal volumes should be tempered by the apparently contradictory findings, or lack of findings, for the structure in autism.
Because the adults with autistic disorder and the comparison subjects were not well matched on IQ, one might speculate that the differences observed in the adults with autistic disorder covary with IQ rather than diagnosis. In our opinion, two factors weigh against this interpretation. First, although IQ was not strictly matched between groups, there were no significant correlations between IQ variables and the volumetric measures. Second, since there have been previous reports of positive correlations between IQ and hippocampal volume, one would expect that the finding, if any, should have been the reverse (i.e., smaller hippocampi in the adults with autistic disorder, relative to the comparison subjects, who had higher IQs). Finally, it is worth noting with respect to IQ that previous population-based studies have indicated a higher mean IQ level in the Denver metropolitan region than in the national standardization sample for the Wechsler scales (37), most likely due to the higher education level in the region, compared to national demographics (38).
To our knowledge, there is only one previous study suggesting larger hippocampal volume in autism (13) (however, see Table 1, footnote “b”). As previously discussed, there are also negative findings with respect to hippocampal volumes in autism (7, 8), one report of smaller hippocampal volume (11), and one report of smaller subregional volume within the hippocampus proper (12). In our view, the hippocampal segmentation criteria used in the current study are most similar to those used in the studies by Aylward et al. (11) and Sparks et al. (13). This similarity leads us to speculate that differences between the studies may be due to differences in the subject populations. A very broad range of functioning among the subjects with autistic disorder was typical in the studies, and one of the two studies reporting smaller volume had the highest functioning participants among the studies (11), so it seems unlikely that IQ could explain the differences. Seizure history was apparently not a factor in the two studies that examined participants with autistic disorder with and without seizure disorders (7, 12). The subjects with autistic disorder in all of the studies reporting negative findings or smaller hippocampal volume had a broader age range of participants with autistic disorder, including children and adults (7–9, 11, 12), than the one study reporting possible larger volume (13), which had the most restricted age range of the studies and included only young children. The current study, however, reports larger hippocampi in an autistic disorder group entirely composed of adults. Sparks et al. (13) have commented on the possibility that the inclusion of both child and adult autistic disorder participants, who are at different stages of hippocampal development and may have different pathological processes contributing to that development, contributes to these differing findings. Finally, while statistical power for the analysis comparing autistic disorder subjects and comparison subjects was relatively good (e.g., Cohen’s d=1.35 for the left hippocampus), the analysis comparing the parents and the comparison subjects yielded a more moderate effect (d=0.55 for the left hippocampus). It is therefore possible that the differences between this study and those listed in Table 1 might also be due to chance.
In the present study, we found smaller left amygdala volume in adults with autistic disorder, relative to parents of children with autistic disorder and the comparison subjects, only in analyses that were adjusted for both total brain volume and gender. Adjustment for gender was particularly necessary in this comparison because of the failure to gender-match the adults with autistic disorder to the other two groups. Two previous studies have reported smaller amygdala volume in autism (11, 17), but Haznedar et al. (10) reported no differences in subjects with autism, and three studies have reported larger amygdala volume in autism (9, 13, 19). Although it is clear that the subject populations and morphometric criteria differ among these studies, in our view no clear pattern emerges to explain the discrepancies among the studies. Statistical power, however, for the studies listed in Table 1 and for the current study is low to moderate, which suggests that much larger groups of subjects may be needed to conclusively answer this question.
The ambiguity surrounding hippocampal volume in autism has implications for the interpretation of the finding of larger hippocampal volumes in the parent group in this study. Assuming that the true direction of volumetric difference is toward larger volumes, the current findings might be interpreted as evidence in support of familiality and possibly a potential genetic basis for hippocampal pathology. If, however, there is no volume difference or smaller volumes in probands, then a genetic explanation seems less likely. For the amygdala findings, since there was no suggestion of a difference between the comparison subjects and the parents of children with autistic disorder, it is less likely that differences in this structure are familial. It should be noted that studies of unaffected parents cannot discriminate genetic from nongenetic familiality—twin studies would be necessary to make this differentiation.
In light of several published papers suggesting larger brain volumes in autism (e.g., references 39–42, but see also reference 43 for negative findings), it is worth commenting on our lack of findings with respect to total brain volume in this study. Recently, Aylward et al. (44) reported evidence that brain size findings in autism are age-dependent, with autistic children showing larger than normal brains and adolescents and adults showing no difference in brain size, relative to age-matched comparison subjects. The authors suggested that autism is characterized by early brain overgrowth, consistent with the findings of Courchesne et al. (41), but that there is a later decrease in brain size for people with autistic disorder at the same time that normal increases in size are seen in typically developing individuals. Thus, in adults with autism, if Aylward et al. (44) are correct, one should not see differences in brain volume, relative to age-matched comparison subjects, which is consistent with the findings of this study.
We believe that this study is the first to report hippocampus and amygdala volumes in parents of autistic disorder probands. This approach has an advantage in that the issues of disorder severity, course, and treatment are simplified by studying clinically unaffected individuals. However, the interpretation of results from studies that include unaffected relatives will remain confusing until a clearer picture emerges concerning the nature of the deficit, if any, in affected individuals. The possibility for age-specific changes in hippocampal and amygdala volume in autism, similar to those described for total brain volume, should be explored in future studies.
 |
 |
 |
Presented in part at the annual meeting of the Society of Biological Psychiatry, San Francisco, May 15–17, 2003. Received May 5, 2003; revision received Dec. 30, 2003; accepted Feb. 10, 2004. From the Department of Psychiatry, University of Colorado Health Sciences Center, Denver. Address reprint requests to Dr. Rojas, Box C268-68 CPH, 4200 E. 9th Ave., Denver, CO 80262; [email protected] (e-mail). Supported by grant PO1 35468 from the Collaborative Programs of Excellence in Autism project of the National Institute of Child Health and Human Development (Dr. Rogers) and by the National Alliance for Autism Research (Dr. Rojas).
1. Bauman M, Kemper TL: Histoanatomic observations of the brain in early infantile autism. Neurology 1985; 35:866–874Crossref, Medline, Google Scholar
2. Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL: Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med 1988; 318:1349–1354Crossref, Medline, Google Scholar
3. Gaffney GR, Kuperman S, Tsai LY, Minchin S: Forebrain structure in infantile autism. J Am Acad Child Adolesc Psychiatry 1989; 28:534–537Crossref, Medline, Google Scholar
4. Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J: An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry 1999; 23:613–624Crossref, Medline, Google Scholar
5. Damasio AR, Maurer RG: A neurological model for childhood autism. Arch Neurol 1978; 35:777–786Crossref, Medline, Google Scholar
6. Bauman M, Kemper TL: Neuroanatomic observations of the brain in autism, in The Neurobiology of Autism. Edited by Bauman M, Kemper TL. Baltimore, Johns Hopkins University Press, 1994, pp 119–145Google Scholar
7. Saitoh O, Courchesne E, Egaas B, Lincoln AJ, Schreibman L: Cross-sectional area of the posterior hippocampus in autistic patients with cerebellar and corpus callosum abnormalities. Neurology 1995; 45:317–324Crossref, Medline, Google Scholar
8. Piven J, Bailey J, Ranson BJ, Arndt S: No difference in hippocampus volume detected on magnetic resonance imaging in autistic individuals. J Autism Dev Disord 1998; 28:105–110Crossref, Medline, Google Scholar
9. Howard MA, Cowell PE, Boucher J, Broks P, Mayes A, Farrant A, Roberts N: Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport 2000; 11:2931–2935Crossref, Medline, Google Scholar
10. Haznedar MM, Buchsbaum MS, Wei T-C, Hof PR, Cartwright C, Bienstock CA, Hollander E: Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry 2000; 157:1994–2001Link, Google Scholar
11. Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD: MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology 1999; 53:2145–2150Crossref, Medline, Google Scholar
12. Saitoh O, Karns CM, Courchesne E: Development of the hippocampal formation from 2 to 42 years: MRI evidence of smaller area dentata in autism. Brain 2001; 124(pt 7):1317–1324Google Scholar
13. Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR: Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002; 59:184–192Crossref, Medline, Google Scholar
14. Lord C, Rutter M, Le Couteur A: Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659–685Crossref, Medline, Google Scholar
15. Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M: The Autism Diagnostic Observation Schedule—Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30:205–223Crossref, Medline, Google Scholar
16. Schopler E, Reichler RJ, DeVellis RF, Daly K: Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J Autism Dev Disord 1980; 10:91–103Crossref, Medline, Google Scholar
17. Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E: Face processing occurs outside the fusiform “face area” in autism: evidence from functional MRI. Brain 2001; 124(pt 10):2059–2073Google Scholar
18. Wing L: The Wing Autism Diagnostic Interview Checklist, in Preschool Children With Inadequate Communication. Edited by Rapin I. Cambridge, UK, Cambridge University Press, 1996, pp 247–251Google Scholar
19. Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Happe F, Frith C, Frith U: The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport 1999; 10:1647–1651Crossref, Medline, Google Scholar
20. Piven J, Bailey J, Ranson BJ, Arndt S: No difference in hippocampus volume detected on magnetic resonance imaging in autistic individuals. J Autism Dev Disord 1998; 28:105–110; correction, 28:271Crossref, Medline, Google Scholar
21. Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M: Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 1995; 25:63–77Crossref, Medline, Google Scholar
22. Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, Tourville J, Kennedy D, Makris N, Caviness VS, Tsuang MT: Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry 1999; 46:941–954Crossref, Medline, Google Scholar
23. Faraone SV, Seidman LJ, Kremen WS, Kennedy D, Makris N, Caviness VS, Goldstein J, Tsuang MT: Structural brain abnormalities among relatives of patients with schizophrenia: implications for linkage studies. Schizophr Res 2003; 60:125–140Crossref, Medline, Google Scholar
24. Narr KL, van Erp TG, Cannon TD, Woods RP, Thompson PM, Jang S, Blanton R, Poutanen VP, Huttunen M, Lonnqvist J, Standerksjold-Nordenstam CG, Kaprio J, Mazziotta JC, Toga AW: A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiol Dis 2002; 11:83–95Crossref, Medline, Google Scholar
25. First MB, Gibbon M, Williams JBW, Spitzer RL: SCID Screen Patient Questionnaire—Extended. North Tonawanda, NY, Multi Health Systems, 1999Google Scholar
26. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for Axis I DSM-IV Disorders: Patient Edition (SCID-I/P). New York, New York State Psychiatric Institute, Biometrics Research, 1994Google Scholar
27. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry 1978; 35:773–782Crossref, Medline, Google Scholar
28. Wechsler D: Wechsler Adult Intelligence Scale—Revised Manual. San Antonio, Tex, Psychological Corp, 1981Google Scholar
29. Wechsler D: Wechsler Adult Intelligence Scale, 3rd ed, Manual. San Antonio, Tex, Psychological Corp, 1997Google Scholar
30. Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G: Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology 1992; 42:1743–1750Crossref, Medline, Google Scholar
31. Honeycutt NA, Smith PD, Aylward E, Li Q, Chan M, Barta PE, Pearlson GD: Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Res 1998; 83:85–94Crossref, Medline, Google Scholar
32. Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL: Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res 1997; 75:31–48Crossref, Medline, Google Scholar
33. Reiss AL, Lee J, Freund L: Neuroanatomy of fragile X syndrome: the temporal lobe. Neurology 1994; 44:1317–1324Crossref, Medline, Google Scholar
34. Hagerman RJ, Cronister A (eds): Fragile X Syndrome: Diagnosis, Treatment, and Research, 2nd ed. Baltimore, Johns Hopkins University Press, 1996Google Scholar
35. Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT: Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA 1997; 94:5401–5404Crossref, Medline, Google Scholar
36. Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT: Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet 2001; 98:161–167Crossref, Medline, Google Scholar
37. Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, Olson RK: A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. J Abnorm Psychol 2001; 110:157–172Crossref, Medline, Google Scholar
38. Bauman KJ, Graf NL: Educational Attainment:2000. Washington, DC, US Census Bureau, 2003Google Scholar
39. Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P: An MRI study of brain size in autism. Am J Psychiatry 1995; 152:1145–1149Link, Google Scholar
40. Piven J, Arndt S, Bailey J, Andreasen N: Regional brain enlargement in autism: a magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 1996; 35:530–536Crossref, Medline, Google Scholar
41. Courchesne E, Carper R, Akshoomoff N: Evidence of brain overgrowth in the first year of life in autism. JAMA 2003; 290:337–344Crossref, Medline, Google Scholar
42. Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY: Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 2001; 57:245–254Crossref, Medline, Google Scholar
43. Garber HJ, Ritvo ER: Magnetic resonance imaging of the posterior fossa in autistic adults. Am J Psychiatry 1992; 149:245–247Link, Google Scholar
44. Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N: Effects of age on brain volume and head circumference in autism. Neurology 2002; 59:175–183Crossref, Medline, Google Scholar



