The Effects of Methylphenidate on Neural Systems of Attention in Attention Deficit Hyperactivity Disorder
Abstract
OBJECTIVE: Recent studies have suggested that attention deficit hyperactivity disorder (ADHD) is associated with abnormalities in basal ganglia and prefrontal cortical functioning. However, these studies have primarily relied upon cognitive tasks that reflect impulse control rather than attentional mechanisms. METHOD: The authors used functional magnetic resonance imaging to investigate the neural correlates of selective and divided attention in a randomized, double-blind, placebo-controlled pharmacological challenge with methylphenidate in 15 adolescents with ADHD (ages 14–17), eight adolescents with reading disorder (ages 12–17), and four adolescents with both reading disorder and ADHD (ages 14–18) who were scanned during both a methylphenidate and a placebo session. Fourteen healthy comparison subjects (ages 12–20) who were not given methylphenidate served as the primary comparison group. RESULTS: During the divided attention task, unmedicated subjects with ADHD or reading disorder recruited the left ventral basal ganglia significantly less than the healthy comparison subjects. Methylphenidate led to an increase in activation in this region but had no effect on task performance. Subjects with ADHD also recruited the middle temporal gyrus significantly less than the comparison subjects, but methylphenidate did not have a direct effect on activation in this region. CONCLUSIONS: These results suggest that ADHD is associated with abnormal processing in attentional networks, with specific dysfunction in striatal circuitry. Methylphenidate may act to normalize activity within this network.
Attention deficit hyperactivity disorder (ADHD) is characterized by inattention, impulsivity, and hyperactivity. Official estimates suggest that 3%–5% of school-age children are affected by this disorder, although estimates range higher than 11% (1). Symptoms often persist into young adulthood, and long-term consequences include lower educational and occupational achievement and increased risk for developing other psychiatric disorders (2, 3).
Several lines of evidence suggest that ADHD is characterized by cognitive deficits in attention and inhibitory processes. Neuropsychological studies have demonstrated that children with ADHD often perform poorly on tasks of frontal lobe and executive functioning, such as the Wisconsin Card Sorting Test (4), the Tower of Hanoi (5), and the Continuous Performance Task (6), and studies measuring event-related potentials have demonstrated frontal abnormalities during executive functioning tasks (7, 8).
Recent neuroimaging studies have supported the notion of frontal cortical dysfunction in ADHD and have specifically implicated dysfunction within frontal-striatal circuits as a putative mechanism associated with impulsive tendencies of ADHD children. Structural magnetic resonance imaging (MRI) studies have found abnormal volumes in both the frontal lobes (9–11) and basal ganglia (10, 12, 13). In addition, functional imaging studies relying on positron emission tomography and single photon emission computed tomography have demonstrated reduced metabolism in frontal and striatal regions in ADHD (14–16). Most recently, functional MRI (fMRI) studies have demonstrated frontal lobe and striatal dysfunction in ADHD during the performance of inhibitory tasks, including the go/no-go and stop tasks (17–19) and the counting Stroop test (20).
While these studies lead to a converging view regarding the neurobiological foundations of impulsivity in ADHD, most have neglected to address the attentional component of the disorder. It is essential to establish the neural circuitry responsible for attentional deficits in ADHD because inattention is a core feature of the disorder (21). Furthermore, the only fMRI study of the effects of stimulant medication on ADHD (18) did not directly address the medication’s effect on the neural circuitry of attention. Therefore, the current study was designed to investigate three unresolved questions regarding the neurobiological foundations of ADHD. First, what are the neural systems responsible for the attentional deficit in ADHD? Second, does the stimulant methylphenidate modulate the activity of these regions? Finally, are the neural deficits and effects of methylphenidate specific to ADHD, or can similar effects be seen in related developmental disorders, such as reading disorder?
To assess the neural systems engaged during attentional processing, we employed a variation of a selective and divided attention task previously used by us (22) that required participants to view and listen to words and decide (yes or no) whether the words were real English words or pseudowords. We hypothesized that ADHD participants would show less activation in the prefrontal cortex and basal ganglia than the comparison subjects, that methylphenidate would increase activation in these areas, and that the activation would be specific to ADHD.
Method
Subjects
The subjects were 15 adolescents (11 boys and four girls, ages 14–17) who were diagnosed with ADHD, combined type; eight adolescents (six boys and two girls, ages 12–17) who were diagnosed with reading disorder; four adolescents (all boys, ages 14–18) with a primary diagnosis of reading disorder who also met DSM-IV criteria for ADHD; and 14 healthy comparison subjects (seven boys and seven girls, ages 12–20).
Subjects in the ADHD and reading disorder groups were recruited from a large cohort of adolescents who participated in previous attention and reading studies at the Yale Center for the Study of Learning and Attention. DSM-IV diagnoses of ADHD were determined from structured clinical interviews (the Diagnostic Interview Schedule for Children, Version 2.3) (23). Exclusion criteria included the presence of comorbid disorders other than oppositional defiant disorder or conduct disorder. The criteria for reading disorder were met if the average of the word identification and the word attack subtests of the Woodcock-Johnson Psychoeducational Test Battery (24) were below a standard score of 90 (below the 25th percentile) or 1.5 standard error of the mean below the expected reading achievement score when we used the WISC-III (25) full-scale IQ (26). Of the 15 subjects with ADHD only, eight were currently being treated with methylphenidate, and an additional four reported a history of methylphenidate therapy. In addition, four subjects with reading disorder only and three subjects with reading disorder plus ADHD reported previous drug therapy with methylphenidate. Table 1 summarizes the subjects’ demographic and IQ data.
Subjects in the healthy comparison group were recruited from the local area and were free of any history of learning disabilities or psychiatric or neurological problems, as determined through detailed interviews with both the participants and their parents. This study was approved by the Yale School of Medicine Human Investigations Committee, and all subjects gave informed written consent for their participation.
Testing Procedure
Each subject in the ADHD and reading disorder groups was tested during two sessions—once when given methylphenidate and once when given placebo. The study was conducted in a double-blind crossover fashion, with testing sessions approximately 1 week apart and the order of sessions counterbalanced across subjects. Approximately 1.25 hours before imaging, the subjects were given methylphenidate hydrochloride or placebo (lactose), with the dose for each subject adjusted for weight with the following guidelines: under 30 kg=15 mg, 30 to 60 kg=20 mg, more than 60 kg=25 mg.
Because of the ethical considerations of giving methylphenidate to healthy individuals, subjects in the comparison group did not participate in the medication trial.
fMRI Tasks
The subjects performed two attentional tasks in the fMRI—a selective attention task and a divided attention task. The selective attention task involved four experimental conditions: visual simple, visual complex, auditory simple, and auditory complex; the divided attention task consisted of one condition. An additional task involving simple button-press responses served as a baseline measure. The task conditions were presented in a block design, with four trials (4.5 seconds each) per block and 13 blocks per fMRI run.
In the selective attention task (Figure 1), a drawing of an eye or an ear (shown for 500 msec) cued the participant to attend to either the visual or auditory stimulus. After a 500-msec pause, a “target” word or pseudoword was presented in the cued modality, i.e., either projected on a screen (visual) or played through headphones (auditory). The subjects made a word/nonword judgment (i.e., yes/no lexical decision) to the target and responded with an appropriate button press. In the visual simple and auditory simple conditions, a nonlinguistic stimulus (a line array or a tone stimulus) was simultaneously presented in the unattended modality. In the visual complex and auditory complex conditions, a word or nonword distracter was simultaneously presented in the unattended modality. Thus, in the complex selective attention conditions (visual complex, auditory complex), the subjects were required to ignore a potentially confusing stimulus in the unattended modality.
In the divided attention task (Figure 1), visual and auditory linguistic stimuli (words or nonwords) were presented simultaneously for 500 msec. After a 500-msec pause, a drawing of both an eye and an ear appeared on the screen for 500 msec, with the eye representing the visual modality and the ear representing the auditory modality. A circle appeared around both, one, or neither of the pictures. The circles were described as a “prediction” the computer gave as to whether the preceding stimulus in each modality was a real word, with the presence of a circle representing a real word. The participants determined whether the computer’s predictions were correct and responded with appropriate button presses.
The baseline control task consisted of button presses with no lexical decision. For selective attention runs, the subjects were shown a picture of a hand with a finger pointing to the right or left for 500 msec. After a 500-msec pause, a line array and a tone were presented simultaneously for 500 msec. The participants were instructed to press the button corresponding to the direction of the finger pointing. For divided attention runs, the line array and tone were presented first, followed by the hand, to better represent the stimulus presentation order for that task.
To minimize the effect of practice from one session to another, two versions of the tasks were created, each containing different sets of stimuli. The subjects were presented with one version during their first session and the other during the second session, with the order counterbalanced across subjects, independent of the random assignment of drug order.
fMRI Data Collection
fMRI scans were acquired with a 1.5-T GE LX MRI scanner (General Electric, Milwaukee) equipped with gradients for echo planar (blood-oxygen-level-dependent [BOLD]) imaging. T1-weighted anatomic images were collected in the sagittal plane by using conventional parameters, followed by 14 axial-oblique slices parallel to the anterior-posterior commissural (AC-PC) line. Functional images were acquired during eight scanning runs: six selective attention runs and two divided attention runs. The additional selective attention runs ensured that an equivalent amount of data was collected for all conditions of the tasks. Stimuli were presented by using PsyScope software and back-projected from a liquid crystal display panel onto a screen viewed by the subject through a prism mirror. The subjects responded by using a magnet-compatible button box. Echo planar imaging parameters were the following: fourteen 7-mm-thick slices parallel to the AC-PC line, TR=1500 msec, TE=60 msec, flip angle=60°, in-plane resolution=3.12×3.12 mm, acquisition matrix=64×64 pixels over a field of view of 20×20 cm.
fMRI Data Analysis
Before statistical analysis, the images from each run were motion-corrected by using the SPM 99 program. Images were discarded if the motion exceeded 2 mm of displacement or 3° of rotation in any direction. In addition, the first two images of each block were discarded to account for the delay in the hemodynamic response. The remaining images were thresholded (with the signal outside the brain set to zero) and Gaussian-filtered (full width at half maximum=6.3 mm). Statistical parametric maps of BOLD activation for each subject were created by using a skew-corrected percent signal difference for each attention task relative to its control task.
Anatomical images and activation maps from individual subjects were transformed into standardized Talairach space (27), and the resulting maps from all subjects in each diagnostic category were superimposed to create cluster-filtered (10 contiguous pixels) composite activation maps for each of the five attention tasks. The probability that the mean percent signal change across subjects was significantly different from zero was calculated by using a t test at each composite pixel. Contrast maps were then created to examine the activation differences across medication status (within subjects) and across diagnostic categories (between subjects). Drug contrasts were created by direct statistical comparison of each subject’s activation when given methylphenidate to activation when given placebo. Group contrasts were made by comparing activation of the ADHD subjects to that of the subjects with reading disorder and of the ADHD and reading disorder subjects relative to the comparison subjects. To account for subjects with combined diagnoses of reading disorder and ADHD, two separate analyses were performed: one in which reading disorder plus ADHD subjects were included in the reading disorder group and another in which these subjects were included in the ADHD group. No differences between the two analyses were observed.
Results
Behavioral Performance
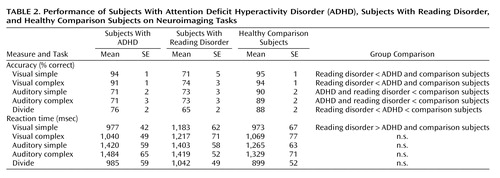
Accuracy (percent correct) and reaction time were monitored during the fMRI session to assess performance for each group (Table 2). To assess the effects of medication in the ADHD and reading disorder groups, reaction times and accuracy for methylphenidate and placebo sessions were compared by using t tests. No effects of medication were seen in either group. Performance data for the ADHD and reading disorder groups were then collapsed across medication status, and multivariate analyses of variance (MANOVAs) (with performance and reaction time on each task as dependent variables and diagnostic status as the independent variable) compared the performance of ADHD, reading disorder, and comparison participants for each of the task conditions.
For accuracy, group differences were found in all five task conditions (F=30.7, df=2, 52, p<0.001 for visual simple; F=25.8, df=2, 52, p<0.001 for visual complex; F=23.1, df=2, 52, p<0.001 for auditory simple; F=12.7, df=2, 52, p<0.001 for auditory complex; and F=22.3, df=2, 52, p<0.001 for divide). Post hoc tests (Tukey’s) revealed that both the comparison subjects and the ADHD subjects performed better than the subjects with reading disorder in the visual selective conditions (p<0.001). In addition, the comparison subjects performed better than either the ADHD or the reading disorder subjects in the auditory selective and divide conditions (p<0.001). Furthermore, in the divide condition, the ADHD subjects performed better than the reading disorder subjects (p<0.005). Analyses of the effect sizes revealed a partial η2 of 0.56 for visual simple, 0.51 for visual complex, 0.49 for auditory simple, 0.34 for auditory complex, and 0.48 for divide. For reaction time, the only performance difference was for the visual simple condition, in which the subjects with reading disorder responded slower than either the comparison subjects or the ADHD subjects (p<0.05 for both).
Imaging Data
Neuroimaging data were remarkably similar for all groups, irrespective of medication or diagnostic status. Regions of significant signal increase associated with selective and divided attention represented a widely distributed network in the brain. Primary regions of activation included the medial superior frontal gyrus (Brodmann’s area 6), anterior cingulate cortex (Brodmann’s areas 32 and 24), dorsolateral prefrontal cortex (Brodmann’s areas 9 and 46), premotor cortex (Brodmann’s area 6), Broca’s area (Brodmann’s area 44), and extrastriate cortex (Brodmann’s areas 18 and 19). In addition, the task conditions requiring attention to the audio modality recruited the primary auditory cortex in the superior temporal gyrus (Brodmann’s area 22). Furthermore, the divided attention task engaged the posterior parietal cortex (Brodmann’s areas 19 and 40) and the basal ganglia. Figure 2 shows activation maps for the divided attention task.
In the divided attention condition, ADHD (and reading disorder) subjects when given methylphenidate recruited the left inferior aspect of the basal ganglia (in the dorsal striatum), but when given placebo, ADHD (and reading disorder) subjects did not recruit this region (Figure 2 and Figure 3). Contrasting the activation of the comparison subjects with ADHD subjects when they were given placebo revealed that comparison subjects recruited this region to a greater extent than the unmedicated ADHD subjects (Figure 3). However, no difference in activation was found in this region when we compared healthy subjects with ADHD subjects when the latter were given methylphenidate (Figure 3). Similar results were observed when we compared healthy subjects to reading disorder subjects but at a lower threshold (Figure 3). In addition to the basal ganglia, the comparison subjects recruited the posterior aspect of the middle temporal gyrus (Brodmann’s area 21) to a greater extent than the ADHD subjects (Figure 3, center). For between-group activation differences in the striatum, analysis of the effect size revealed a partial η2 of 0.24. The activation differences in the striatum were not correlated with either measure of task performance (r=0.08 for accuracy, r=–0.10 for reaction time). Temporal lobe activation was correlated only with accuracy for the visual selective tasks and only in the left hemisphere (r=0.33, p=0.01 for visual simple; r=0.32, p<0.05 for visual complex). Additionally, these activations were not correlated with IQ (r=0.08).
Discussion
These results indicate that unmedicated ADHD adolescents differed from healthy comparison subjects in the activation of the left ventral aspect of the basal ganglia during the performance of a divided attention task. Specifically, the comparison subjects activated this region to a greater extent than the ADHD participants when given placebo. When the ADHD adolescents were given a challenge dose of methylphenidate before scanning, they recruited this region of the basal ganglia to a similar degree as the comparison subjects. Similarly, unmedicated reading disorder adolescents showed a reduction in activation of the left striatum relative to healthy comparison subjects during the divided attention task, and a challenge dose of methylphenidate normalized activation in this region. Although performance on the task differed between the ADHD, reading disorder, and comparison adolescents, neither measure of performance was correlated with activation in the striatum. Therefore, these activation differences cannot be accounted for simply by performance differences. Additionally, the comparison subjects recruited the posterior aspect of the middle temporal gyrus to a greater extent than the ADHD adolescents during the attention tasks. These differences also appear to be independent of any consistent pattern in task performance. The lack of a correlation between IQ and striatal activation suggests that any IQ differences between the ADHD and reading disorder adolescents did not significantly affect our neuroimaging findings.
Our results indicate that adolescents with either ADHD or reading disorder show quite similar neural activation patterns to healthy comparison subjects during the performance of selective and divided attention tasks. For all subjects, the selective and divided attention tasks recruited a host of cortical areas, including the dorsolateral prefrontal cortex, the anterior cingulate cortex, the premotor cortex, and Broca’s area and, particularly for divided attention, the posterior parietal area and regions of the basal ganglia. These findings serve to reinforce the role of these cortical and subcortical structures in the processing of attention-related information and the generation of behavioral responses and indicate that adolescents with either ADHD or reading disorder successfully recruit the majority of these regions.
Our findings of reduced striatal activation for adolescents with ADHD are consistent with prior neuroimaging studies showing that ADHD subjects exhibit less activity in basal ganglia structures both at rest and during the performance of cognitive tasks (14, 15, 18, 19, 28). Moreover, our finding that methylphenidate normalized striatal activation is consistent with previous reports that methylphenidate preferentially modulates striatal activity in ADHD subjects (14, 15, 18) and increases extracellular dopamine in the striatum in healthy adults (29). While we did not observe differences in frontal cortical functioning, this finding is supported by prior fMRI results showing that frontal lobe activity may be more related to task parameters than to either diagnostic or medication status (18). A lack of consistent neuropsychological findings in children and adolescents with ADHD (30) supports the notion that frontal cortical deficits in ADHD may be subtle and related only to specific components of executive functioning.
An intriguing finding in the current study is that methylphenidate increased striatal activation for both the ADHD and reading disorder adolescents. These results suggest that methylphenidate may have similar modulatory effects in certain brain regions, whether or not an attention disorder is present. Our findings fit well with earlier research demonstrating that low-dose psychostimulants can enhance cognitive performance and reduce impulsiveness in individuals other than those with ADHD, including normal children and adults (31, 32).
In our study, methylphenidate significantly increased striatal activity only during the divided attention task. This task specificity is not surprising after accounting for the neural structures engaged by the various task conditions. Relative to the baseline sensorimotor task, the basal ganglia were preferentially activated only by the divided attention task. One interpretation of this pattern is that the divided attention condition required additional cognitive processing over the selective attention conditions. More specifically, the selective attention conditions required a two-stage cognitive process in which the participant first encoded the auditory and visual stimuli and then made a judgment as to whether the stimulus in the attended modality was a real word or a pseudoword. In the divided attention task, an additional step required participants to determine the accuracy of a prediction by the computer as to the lexical nature of the previously presented stimuli.
It is reasonable to suggest that the selective attention conditions, when directly compared to the baseline task, did not strongly engage the basal ganglia because these tasks were not demanding enough to require additional processing afforded by basal ganglia–cortical circuitry. Because the divided attention task was designed to strongly engage executive processes, that task led to increased activation of the basal ganglia relative to the sensorimotor task. Therefore, we suggest that methylphenidate modulated striatal activity only during the divided attention task because it was during this task that these structures were preferentially activated.
The current findings provide novel information about the regional neural effects of methylphenidate during attentional control. However, the mechanisms by which the actions of methylphenidate translate into the improvement often seen in the cognitive and behavioral symptoms associated with ADHD remain unknown. The enhanced activation of the striatum by methylphenidate may reflect an increase in neural processing related to the inhibition of prepotent or impulsive responses (18) or the selection and execution of appropriate behavioral responses (33). Alternatively, methylphenidate-induced increases in dopamine activity within the striatum may increase the motivational salience (29, 34) of the task without necessarily making the task easier to perform. Our results are consistent with both interpretations.
The role of the reduced activation in temporal lobe regions observed in ADHD adolescents is more difficult to determine. There is little prior evidence suggesting that ADHD is associated with abnormalities in temporal cortical circuitry. However, our findings of reduced activation of the middle temporal gyrus in ADHD participants were quite robust, particularly for the auditory selective tasks. The region of the middle temporal gyrus in which these differences occurred lies in the same area of the temporal lobe associated with neural deficits in dyslexia (26). We suggest that this region of the middle temporal gyrus mediates attentional processing for verbal stimuli and may be particularly important when attending to auditory information. Further investigation into the role of the middle temporal gyrus in attention and ADHD is warranted.
In summary, our findings demonstrate neural dysfunction and neural effects of methylphenidate in adolescents with ADHD or reading disorder during the performance of attention tasks. While our ADHD group was larger than in previous fMRI studies of ADHD, the current study was potentially limited by the small size of the group with reading disorder only. The size of our reading disorder group, though, is consistent with many reported fMRI studies, particularly those involving clinical populations. Moreover, our imaging results demonstrating striatal dysfunction for both ADHD and reading disorder participants indicate that we had sufficient power to draw meaningful conclusions from the reading disorder group. Nevertheless, replication of this study with a larger reading disorder group would reveal with more certainty whether neural deficiencies underlying poor attentional control are specific to ADHD or are observed as well in other developmental disorders.
We also note that most of the ADHD subjects and a number of the reading disorder subjects had a history of drug therapy with methylphenidate before this study. While we attempted to control for medication history by ensuring that participants were medication free for at least 72 hours before testing, this does not eliminate possible long-term modulation of neural functioning stemming from methylphenidate use. Thus, it is unclear whether any additional neural effects of methylphenidate would be seen for ADHD subjects with no prior history of medication use. Despite these limitations, our findings are consistent with current conceptions of ADHD and provide novel insights into the neural mechanisms underlying the effect of methylphenidate on attentional control.
 |
 |
Received Sept. 5, 2003; revision received Jan. 16, 2004; accepted Feb. 4, 2004. From the Brain Imaging and Analysis Center, Duke University Medical Center; the Interdepartmental Neuroscience Program, the Department of Pediatrics, and the Department of Neurology, Yale University School of Medicine, New Haven, Conn.; and the Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, Tenn. Address reprint requests to Dr. Shafritz, Brain Imaging and Analysis Center, Duke University Medical Center, Box 3918, Durham, NC 27710; [email protected] (e-mail). Supported by grants from the National Institute of Child Health and Human Development (PO1-HD-21888 and P50-HD-25802) and the National Institute for Biomedical Imaging and Bioengineering (EB-000461). The authors thank Hedy Sarofin, Terry Hickey, and Cheryl McMurray for technical assistance; Kenneth Pugh and W. Einar Mencl for help with task design and behavioral data analysis; Cheryl Lacadie and Pawel Skudlarski for help with analysis of functional magnetic resonance imaging data; Donna Lutz for commentary; and the Yale Center for Learning and Attention for assistance in testing study participants.
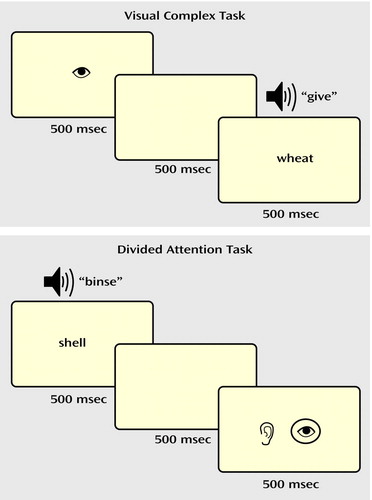
Figure 1. Trial Design for Visual Complex and Divided Attention Tasks Performed by Subjects With Attention Deficit Hyperactivity Disorder (ADHD), Subjects With Reading Disorder, and Healthy Comparison Subjectsa
aIn selective attention trials, subjects judged whether a verbal stimulus in the cued modality was a real word or a pseudoword. In divided attention trials, stimuli (words or pseudowords) were presented simultaneously in both modalities, and subjects judged whether a prediction of the lexical nature of the words was correct.
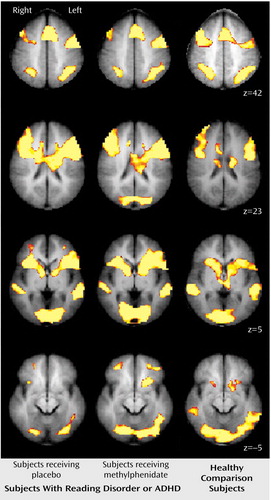
Figure 2. Composite Maps Demonstrating Brain Activation for the Divided Attention Task Performed by Subjects With Either Attention Deficit Hyperactivity Disorder (ADHD) or Reading Disorder, and Healthy Comparison Subjectsa
aComposite images obtained from subjects with either ADHD or reading disorder when they were given placebo (left) and methylphenidate (center) are shown beside those obtained from healthy comparison subjects (right). Red-yellow indicates brain regions that were significantly more activated during the attention task than the baseline task (p<0.005, uncorrected). Positions (in millimeters) along the z axis in Talairach space are shown to the right.
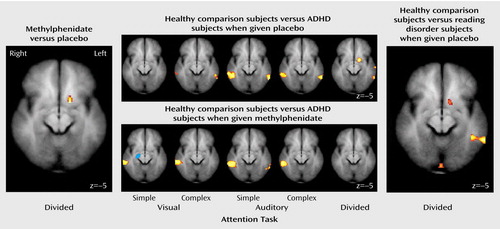
Figure 3. Drug Contrast and Group Contrast Maps Directly Comparing Activation During the Divided Attention Task for Subjects With Attention Deficit Hyperactivity Disorder (ADHD), Subjects With Reading Disorder, and Healthy Comparison Subjectsa
aIn the comparison of adolescents with either ADHD or reading disorder when they received methylphenidate versus when they received placebo, red-yellow indicates regions that were significantly more activated when subjects received methylphenidate than when subjects received placebo (p<0.01, uncorrected). In the comparison of healthy subjects to ADHD subjects when they received placebo, regions of greater activation in the healthy comparison subjects are shown in red-yellow (p<0.01, uncorrected). In the comparison of healthy subjects to ADHD subjects when they received methylphenidate, regions of greater activation in the comparison subjects are shown in red-yellow and regions of greater activation in the ADHD subjects are shown in blue (p<0.01, uncorrected). In the comparison of healthy subjects to subjects with reading disorder when they received placebo, regions of greater activation in the healthy comparison subjects are shown in red-yellow (p<0.02, uncorrected). Positions (in millimeters) along the z axis are shown in the lower right of each map.
1. Wolraich ML, Hannah JN, Pinnock TY, Baumgaertel A, Brown J: Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a county-wide sample. J Am Acad Child Adolesc Psychiatry 1996; 35:319–324Crossref, Medline, Google Scholar
2. Mannuzza S, Klein RG, Bessler A, Malloy P, Hynes ME: Educational and occupational outcome of hyperactive boys grown up. J Am Acad Child Adolesc Psychiatry 1997; 36:1222–1227Crossref, Medline, Google Scholar
3. Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M: Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry 1998; 155:493–498Link, Google Scholar
4. Seidman LJ, Biederman J, Faraone SV, Weber W, Ouellette C: Toward defining a neuropsychology of attention-deficit hyperactivity disorder: performance of children and adolescents from a large clinically referred sample. J Consult Clin Psychol 1997; 65:150–160Crossref, Medline, Google Scholar
5. Klorman R, Hazel-Fernandez LA, Shaywitz SE, Fletcher JM, Marchione KE, Holahan JM, Stuebing KK, Shaywitz BA: Executive functioning deficits in attention-deficit/hyperactivity disorder are independent of oppositional defiant or reading disorder. J Am Acad Child Adolesc Psychiatry 1999; 38:1148–1155Crossref, Medline, Google Scholar
6. Grodzinsky GM, Diamond R: Frontal lobe functioning in boys with attention-deficit hyperactivity disorder. Dev Neuropsychol 1992; 8:427–445Crossref, Google Scholar
7. van Leeuwen TH, Steinhausen H-C, Overtoom CCE, Pascual-Marqui RD, van’t Klooster B, Rothenberger A, Sergeant JA, Brandeis D: The Continuous Performance Test revisited with neuroelectric mapping: impaired orienting in children with attention deficits. Behav Brain Res 1998; 94:97–110Crossref, Medline, Google Scholar
8. Brandeis D, van Leeuwen TH, Rubia K, Vitacco D, Steger J, Pascual-Marqui RD, Steinhausen H-C: Neuroelectric mapping reveals precursor of stop failures in children with attention deficits. Behav Brain Res 1998; 94:111–125Crossref, Medline, Google Scholar
9. Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL: Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 2002; 288:1740–1748Crossref, Medline, Google Scholar
10. Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL: Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1996; 53:607–616Crossref, Medline, Google Scholar
11. Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J: Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology 1997; 48:589–601Crossref, Medline, Google Scholar
12. Aylward EH, Reiss AL, Reader MJ, Singer HS, Brown JE, Denkla MB: Basal ganglia volumes in children with attention-deficit hyperactivity disorder. J Child Neurol 1996; 11:112–115Crossref, Medline, Google Scholar
13. Mataro M, Garcia-Sanchez C, Junque C, Estevez-Gonzalez A, Pujol J: Magnetic resonance imaging measurement of the caudate nucleus in adolescents with attention-deficit hyperactivity disorder and its relationship with neuropsychological and behavioral measures. Arch Neurol 1997; 54:963–968Crossref, Medline, Google Scholar
14. Lou HC, Henriksen L, Bruhn P: Focal cerebral hypoperfusion in children with dysphasia and/or attention deficit disorder. Arch Neurol 1984; 41:825–829Crossref, Medline, Google Scholar
15. Lou HC, Henriksen L, Bruhn P, Borner H, Nielsen JB: Striatal dysfunction in attention deficit and hyperkinetic disorder. Arch Neurol 1989; 46:48–52Crossref, Medline, Google Scholar
16. Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, Hamburger S, Cohen RM: Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990; 323:1361–1366Crossref, Medline, Google Scholar
17. Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ: Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 2003; 53:871–878Crossref, Medline, Google Scholar
18. Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JDE: Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 1998; 95:14494–14499Crossref, Medline, Google Scholar
19. Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore ET: Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry 1999; 156:891–896Link, Google Scholar
20. Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J: Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting Stroop. Biol Psychiatry 1999; 45:1542–1552Crossref, Medline, Google Scholar
21. Hales RE, Yudofsky SC, Talbott JA (eds): The American Psychiatric Press Textbook of Psychiatry, 3rd ed. Washington, DC, American Psychiatric Press, 1999Google Scholar
22. Shaywitz BA, Shaywitz SE, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Marchione KE, Fletcher JM, Klorman R, Lacadie C, Gore JC: The functional neural architecture of components of attention in language-processing tasks. Neuroimage 2001; 13:601–612Crossref, Medline, Google Scholar
23. Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, Lahey BB, Bourdon K, Jensen PS, Bird HR, Canino G, Regier DA: The NIMH Diagnostic Interview Schedule for Children, version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the MECA study (Methods for the Epidemiology of Child and Adolescent Mental Disorders Study). J Am Acad Child Adolesc Psychiatry 1996; 35:865–877Crossref, Medline, Google Scholar
24. Woodcock RW, Johnson BB: Woodcock-Johnson Psychoeducational Battery—Revised. Allen, Tex, Developmental Learning Materials, 1989Google Scholar
25. Wechsler D: Wechsler Intelligence Scale for Children. San Antonio, Tex, Psychological Corp, 1991Google Scholar
26. Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC: Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry 2002; 52:101–110Crossref, Medline, Google Scholar
27. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
28. Teicher MH, Anderson CM, Polcari A, Glod CA, Maas LC, Renshaw PF: Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nat Med 2000; 6:470–473Crossref, Medline, Google Scholar
29. Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D: Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001; 21:RC121Google Scholar
30. Barkley RA, Grodzinsky G, DuPaul GJ: Frontal lobe functions in attention deficit disorder with and without hyperactivity: a review and research report. J Abnorm Child Psychol 1992; 20:163–188Crossref, Medline, Google Scholar
31. Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ: Dextroamphetamine: its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry 1980; 37:933–943Crossref, Medline, Google Scholar
32. Solanto MV: Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res 1998; 94:127–152Crossref, Medline, Google Scholar
33. Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC: Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:374–383Crossref, Medline, Google Scholar
34. Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, Pappas N: “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 2002; 44:175–180Crossref, Medline, Google Scholar



