Depression Severity and Drug Injection HIV Risk Behaviors
Abstract
OBJECTIVE: This study examined the association of depression severity and drug injection HIV risk behavior among injection drug users. METHOD: Injection drug users who met the DSM-IV criteria for major depressive disorder, dysthymia, or substance-induced mood disorder lasting at least 3 months were asked how often they used “needles or syringes that someone else had used” (injection risk behavior) in the past 90 days. Depression severity was measured by using the Modified Hamilton Rating Scale for Depression. RESULTS: Of the 109 subjects, 63% were male, 82% were Caucasian, and 10% were HIV positive. The subjects’ mean modified Hamilton depression scale score was 21.0 (SD=3.9). The mean number of reported instances of injection risk behavior (needle sharing) in the past 90 days was 57.5 (SD=134.7). In a logistic regression analysis in which the effects of age, race, gender, number of days on which injection drugs were used (injection days), and average number of injections per injection day were controlled, depression severity was associated with injection risk (odds ratio=1.5; 95% confidence interval=1.1–2.3). CONCLUSIONS: Greater severity of depression is associated with greater frequency of injection risk behavior among depressed injection drug users. Risk reduction programs that target depressed injection drug users need to be designed.
Depressive disorders are common among opiate abusers (1–6). Depression is a frequent internal cue triggering drug craving (7, 8). As comorbid depression is associated with continued drug use, it has been hypothesized that depression is also associated with continued HIV risk-taking through sharing of drug equipment (9, 10). Higher scores on measures of psychological distress, inventories measuring symptoms of depression, and composite measures of depression, anxiety, and hostility have been associated with higher levels of injection-related risk taking (11–14).
Within the last 10 years, nearly half of the new cases of HIV infection in the United States have been among injection drug users (15). Because measures for reducing the occurrence of AIDS associated with injection drug use include encouraging safer injecting practices, depression might have considerable influence as a modifiable factor. Previous reports linking depressive symptoms with injection risk behaviors have lacked diagnostic assessments and have not considered the severity of the depressive symptoms (9–14). This study examines the association of depression severity and injection risk behaviors in a cohort of active drug injectors.
Method
Study Site and Subjects
Between March 2000 and December 2001, we recruited subjects in Providence, R.I., for a randomized study of outpatient treatment of depression for drug injectors in the community.
Subjects were eligible for inclusion if they 1) were ages 18–70 years; 2) had injected opiate or cocaine during the past 30 days; 3) met the criteria for a DSM-IV diagnosis according to the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) (16) of either major depressive disorder, dysthymia, substance-induced mood disorder with symptoms persisting for at least the last 3 months, or major depressive disorder plus dysthymia; 4) had a Modified Hamilton Rating Scale for Depression (17) score ≥14; 5) were willing to transfer their current medication management to the study psychiatrist; 6) did not have a current or past diagnosis of bipolar disorder, schizophrenia, schizoaffective disorder, schizophreniform disorder, or delusional disorder; and 7) were English-speaking.
Eligible subjects who agreed to participate signed a consent form approved by the Rhode Island Hospital Institutional Review Board; were administered structured interviews, including the SCID-P and questionnaires about sociodemographic characteristics, illicit drug use, alcohol use, and HIV risk behaviors; underwent HIV antibody testing; and received $20 reimbursement for their time.
Measures
To assess injection risk behavior, subjects were asked, “In the past 3 months, how many days did you inject drugs with a needle?” and “In the past 3 months, how many times did you inject each day?” (18). We multiplied the numbers they provided to estimate the total number of injections during the 90 days before the baseline interview. Injection risk was measured by asking subjects, “In the past 3 months you said you injected _____ times. How many of these times did you use needles or syringes that someone else had used and were not sterilized or cleaned with bleach before you used them?” We constructed a four-category response variable—no injection risk, one to five instances of injection risk, six to 89 instances, and 90 instances or more—and used analytical methods appropriate for ordinal response variables.
The primary predictor of interest was depression severity, which was assessed by using the modified Hamilton depression scale. Control variables included the total number of injections during the past 90 days; treatment involvement (any residential, outpatient, or methadone treatment or drug detoxification) during the past 90 days; use of benzodiazepines and noninjection cocaine, both of which are predictors of high-risk injecting practices (19–21); and alcohol problems as assessed with the Addiction Severity Index (22).
Analytical Method
We report results from bivariate and multivariate ordered logistic regression models (23). We used the cumulative odds ratio as a measure of effect size. Continuous predictor variables were standardized to zero mean and unit variance before analysis (23). We report z statistics and confidence interval estimates based on robust standard error estimators (Huber-White sandwich estimators) (24).
Results
The majority of the 109 subjects were male (N=69, 63.3%) and Caucasian (N=89, 81.7%). The subjects’ mean age was 36.56 years (SD=8.82), and 10% were HIV positive. The subjects had been injecting drugs for an average of 13.5 years (SD=10.3), and 91% (N=99) reported heroin as their primary injection drug. Injection frequency was not correlated with depression severity (r=0.08, N=109, p=0.40). Just over half of the subjects (N=55; 50.5%) used benzodiazepines, and 61.5% (N=67) reported having used noninjection cocaine. A majority (N=69, 63.3%) of the subjects reported contact with one or more drug treatment modalities in the past 3 months. The mean score on the modified Hamilton depression scale was 20.97 (SD=3.93, range=14–34).
During the 90-day reporting period, subjects reported injecting drugs on an average of 68.92 days (SD=26.03, median=82.00) and averaged 258.73 total instances of drug injection (SD=203.30, median=225.00). The mean number of reported injection risk instances in the past 90 days was 57.51 (SD=134.72, range=0–750). Of the 109 subjects, 31.2% (N=34) reported no injection risk behavior, 26.6% (N=29) reported one to five instances of risk behavior, 27.5% (N=30) reported six to 89 instances, and 14.7% (N=16) reported 90 instances or more.
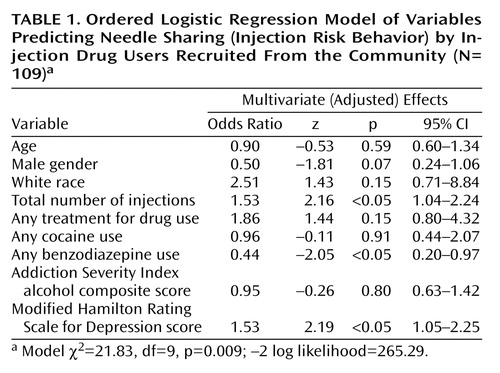
To control for potential confounding, we estimated a model with all nine selected predictor variables (Table 1). The magnitude of the adjusted association between injection risk behavior and the modified Hamilton depression scale score was 1.53 (z=2.19, p<0.05). Because of concerns about the small number of subjects, we used bootstrap resampling (200 repetitions) to estimate empirically based standard errors for the adjusted effect of the modified Hamilton depression scale score; the results were consistent with those reported in Table 1 (z=2.34, p<0.05). After adjustment for other covariates, an increase of one standard deviation in the total number of injections increased the cumulative odds of injection risk behavior by a factor of 1.53 (z=2.16, p<0.05), and benzodiazepine use was associated with lower odds of injection risk behavior (odds ratio=0.44, z=–2.05, p<0.05). Other covariates were not statistically significant predictors of injection risk.
Discussion
Identifying and attempting to modify the predictors of risky injection behavior is one approach to the prevention of transmission of blood-borne viruses. In this study of depressed drug injectors, we found that greater severity of depression was associated with increased sharing of needles and syringes, placing users at risk for HIV and hepatitis. These findings clarify the independent effect of psychiatric severity relative to other sociodemographic factors and frequency of drug injection.
While injection frequency was associated with greater injection risk, depression did not increase injection frequency in the subjects in our study. There are alternative mechanisms by which depression may increase risk taking. First, psychological factors may influence an injector’s perception of the threat posed by an infectious disease, and depression may engender fatalism about HIV, a form of passive suicidal behavior. Second, depression has been reported to be significantly and negatively correlated with drug users’ confidence in giving careful thought to the consequences of life decisions (25); depression could thereby lower the likelihood of taking preventive action. Third, depression may affect an individual’s attention, promoting carelessness in drug use activities. Fourth, depression may index a lesser ability to cope with stressful life events, leading to higher levels of unmindful sharing of drug equipment. Fifth, depression may be serving as a marker of additional psychiatric diagnoses, such as anxiety disorders, that have been associated with drug injection risk (14). Finally, more depressed injectors may report more risk than would objectively be observed.
The subjects in this study included only injection drug users who met the DSM-IV criteria for major depressive disorder(63%), dysthymia (2%), major depressive disorder plus dysthymia (17%), or persistent substance-induced mood disorder (17%). A significant challenge in the study of depression in opiate users is that opiate use may induce transient symptoms that are difficult to distinguish from the symptoms of primary mood disorders (26, 27), despite recent attempts to develop distinctive diagnostic criteria and interview instruments. Previous studies of the association of mood and injection risk taking have assessed only depressive symptoms but have not used formal diagnostic criteria. Symptom scores are likely to be less stable than diagnoses. Nonetheless, our findings suggest that the dichotomous diagnostic approach to depression may not capture the association with HIV risk behaviors that a continuous measure such as the modified Hamilton depression scale offers. It is noteworthy that our study did not include a group of nondepressed injectors who may have a lower frequency of risky injection behavior (9–14), and, as in most studies of injection drug users, the measurement of injection behavior was based on self-report. Finally, although the number of subjects was small, the community-based recruitment and the use of robust standard error estimators should add to confidence in the findings.
Benzodiazepine use was related to a lower rate of injection risk taking, in contrast to the findings of previous studies in which benzodiazepine use was associated with higher rates of injection risk (19, 28). In these previous studies, benzodiazepine use may have been a marker for a particularly dysfunctional subgroup of polydrug users rather than an agent of risk taking. We speculate that for the drug injectors with a diagnosis of depression who were the subjects in our study, benzodiazepine use may have been a marker of social isolation, which allowed fewer opportunities to share drug equipment.
All injectors who have a current axis I diagnosis of depression should be candidates for treatment. The obvious clinical implication of the association reported here is that if depression severity can be reduced, the rate of high-risk injection behaviors may also be reduced. Comorbid depression and substance use are interactive and mutually maintaining. If untreated, depression affects readiness to modify risk behaviors, a basic component of behavioral change; thus, treatment of depression may advance readiness to change. Regardless of the causal relationship that underlies the association of major depression and needle-sharing, risk reduction programs targeted at injection drug users need to be designed with an awareness of the role of psychiatric disorders.
 |
Received Oct. 8, 2002; revision received Feb. 10, 2003; accepted Feb. 22, 2003. From the Department of Medicine, Brown Medical School, Providence, R.I.; and the Division of General Internal Medicine and the Department of Psychiatry, Rhode Island Hospital. Address reprint requests to Dr. Stein, Division of General Internal Medicine, Rhode Island Hospital, 593 Eddy St., Providence, RI 02903; [email protected] (e-mail). Supported by NIMH grants MH-61141 and MH-62719 and Mid-Career Investigator Award KA-00512 to Dr. Stein from the National Institute on Drug Abuse.
1. Croughan JL, Miller JP, Wagelin D, Whitman BY: Psychiatric illness in male and female narcotic addicts. J Clin Psychiatry 1982; 43:225–228Medline, Google Scholar
2. Rounsaville BJ, Weissman MM, Crits-Christoph K, Wilber C, Kleber H: Diagnosis and symptoms of depression in opiate addicts. Arch Gen Psychiatry 1982; 39:151–156Crossref, Medline, Google Scholar
3. Kosten TR, Rounsaville BJ, Kleber HD: Relationship of depression to psychosocial stressors in heroin addicts. J Nerv Ment Dis 1983; 171:97–104Crossref, Medline, Google Scholar
4. Khantzian EJ, Treece C: DSM-III psychiatric disorders of narcotic addicts. Arch Gen Psychiatry 1982; 42:1067–1071Crossref, Google Scholar
5. Kranzler HR, Liebowitz NR: Anxiety and depression in substance abuse: clinical implications. Med Clin North Am 1988; 72:867–885Crossref, Medline, Google Scholar
6. Brienza RS, Stein MD, Chen MH, Gogineni A, Sobota M, Phil M, Maksad J, Hu P, Clarke J: Depression among needles exchange program and methadone maintenance clients. J Subst Abuse Treat 2000; 18:331–337Crossref, Medline, Google Scholar
7. Marlatt GA, Gordon JR: Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Diseases. New York, Guilford, 1985Google Scholar
8. McClellan AT, Luborsky L, O’Brien CP, Woody CE, Druley KA: Predicting response to alcohol and drug treatment: role of psychiatric symptoms. Arch Gen Psychiatry 1983; 40:620–628Crossref, Medline, Google Scholar
9. Woody GE, Metzger D, Navaline H, McLellan T, O’Brien CP: Psychiatric symptoms, risky behavior and HIV infection. NIDA Res Monogr 1997; 172:156–170Medline, Google Scholar
10. Latkin CA, Mandell W: Depression as an antecedent of frequency of intravenous drug use in an urban, nontreatment sample. Int J Addict 1993; 28:1601–1612Crossref, Medline, Google Scholar
11. Metzger D, Woody G, De Philippis D, McLellan AT, O’Brien CP, Platt JJ: Risk factors for needle sharing among methadone-treated patients. Am J Psychiatry 1991; 148:636–640Link, Google Scholar
12. Abbott PJ, Weller SB, Walker SR: Psychiatric disorders of opioid addicts entering treatment: preliminary data. J Addict Dis 1994; 13:1–11Crossref, Medline, Google Scholar
13. Camacho LM, Brown BS, Simpson DD: Psychological dysfunction and HIV/AIDS risk behavior. J Acquir Immune Defic Syndr 1996; 11:198–202Crossref, Google Scholar
14. Maslow RM, Corrigan SA, Pena JM, Calkins AM, Bannister TM: Mood and HIV risk behavior among drug-dependent veterans. Psychol Addict Behav 1992; 6:131–134Crossref, Google Scholar
15. Holmberg SD: The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health 1996; 86:642–654Crossref, Medline, Google Scholar
16. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
17. Miller IW, Bishop S, Norman W, Madderer H: The Modified Hamilton Rating Scale for Depression: reliability and validity. Psychiatry Res 1985; 14:131–142Crossref, Medline, Google Scholar
18. Camacho LM, Batholomew NG, Joe GW, Simpson DD: Maintenance of HIV risk reduction among injection opioid users: a 12 month post-treatment follow-up. Drug Alcohol Depend 1992; 47:11–18Crossref, Google Scholar
19. Darke S, Hall W, Ross M, Wodak A: Benzodiazepine use and HIV risk-taking behaviour among injecting drug users. Drug Alcohol Depend 1992; 31:31–36Crossref, Medline, Google Scholar
20. Doherty MC, Garfein RS, Monterroso F, Brown D, Vlahov D: Correlates of HIV infection among young adult short-term injection drug users. AIDS 2000; 14:717–726Crossref, Medline, Google Scholar
21. Mandell W, Vlahov D, Latkin C, Ozemkowska M, Cohn S: Correlates of needle sharing among injection drug users. Am J Public Health 1994; 84:920–923Crossref, Medline, Google Scholar
22. McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M: The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 1992; 9:199–213Crossref, Medline, Google Scholar
23. Stata Reference Manual: Release 7.0. College Station, Tex, Stata Corp, 2001Google Scholar
24. Long JS: Regression Models for Categorical and Limited Dependent Variables. Thousand Oaks, Calif, Sage Publications, 1997Google Scholar
25. Joe GW, Knezek L, Watson D, Simpson DD: Depression and decision-making among intravenous drug users. Psychiatr Rep 1991; 68:339–347Crossref, Medline, Google Scholar
26. Nunes EV, Goehl L, Seracini A, Deliyannides D, Donovan S, Koenig T, Quitkin FM, Williams JBW: A modification of the structured clinical interview for DSM-III-R to evaluate methadone patients. Am J Addict 1996; 5:241–248Google Scholar
27. Hasin D, Trautman K, Endicott J: Psychiatric research interview for substance and mental disorders: phenomenologically based diagnosis in patients who abuse alcohol and drugs. Psychopharmacol Bull 1998; 34:3–8Crossref, Medline, Google Scholar
28. Darke S, Swift W, Hall W, Ross M: Drug use, HIV risk-taking and psychosocial correlates of benzodiazepine use among methadone maintenance clients. Drug Alcohol Depend 1993; 34:67–70Crossref, Medline, Google Scholar



