Effects of Elevated Serum Prolactin on Bone Mineral Density and Bone Metabolism in Female Patients With Schizophrenia: A Prospective Study
Abstract
OBJECTIVE: This study was designed to prospectively examine differential effects of sustained “high” and “low” serum prolactin levels on bone mineral density and peripheral markers of bone metabolism. METHOD: A dual-energy X-ray absorptiometer was used to measure bone mineral density. Peripheral markers of bone formation and resorption were used to measure bone metabolism in 14 Caucasian female patients with schizophrenia treated with risperidone or olanzapine monotherapy over 12 months. RESULTS: Analyses of variance failed to show an association between elevated prolactin and bone mineral loss over time. However, higher rates of bone formation and resorption were seen in those with high prolactin levels. CONCLUSIONS: The results failed to show that elevated prolactin accelerates bone mineral density loss. However, sustained hyperprolactinemia did have an impact on the rate of bone metabolism. Perhaps higher prolactin levels over longer time periods are necessary before the metabolic processes become uncoupled, leading to bone mineral density loss.
Hyperprolactinemia is often seen among persons with schizophrenia, most commonly as a result of antipsychotic drugs that act as D2 receptor antagonists on pituitary lactotrophs (1). Sustained hyperprolactinemia secondary to prolactin secreting adenomas is known to result in bone mass loss, leading to osteoporosis (2, 3). However, the impact of neuroleptic-induced hyperprolactinemia on bone loss in patients with schizophrenia has largely been ignored (4, 5). To explore this issue, serum prolactin levels, measures of bone mineral density, and peripheral markers of bone metabolism were collected prospectively from 14 female patients with schizophrenia over a 12-month period. Our objectives were to investigate the effects of sustained hyperprolactinemia on bone mineral density and to examine the effects of hyperprolactinemia on bone formation and resorption. Other mediating risk factors were explored.
Method
From an original sample of 20 patients, 14 Caucasian female patients with schizophrenia ranging in age from 22 to 53 years (mean=36.3, SD=9.4) completed this study. Mean duration of illness was 16.4 years (SD=10.1). They remained on regimens of risperidone (N=6) or olanzapine (N=8) monotherapy throughout the 12-month study. Written informed consent was obtained from all subjects. Extensive inclusion and exclusion criteria resulted in a group of patients with DSM-IV schizophrenia who were in good general health and clinically stable after at least 1 month of treatment with either olanzapine or risperidone. In brief, subjects were excluded for any medical condition or treatment known to alter bone mineral density (e.g., Cushing’s syndrome).
Baseline prolactin levels were used to divide the group (median split) into those with high prolactin levels (mean=88.8 ng/ml, SD=34.7) and low prolactin levels (mean=21.1 ng/ml, SD=15.7); all risperidone-treated patients and one olanzapine-treated patient comprised the high prolactin group. Six other noncompliant patients were dropped from the study. All patients were premenopausal with the exception of one patient who was judged to be perimenopausal at the beginning of the study.
A Hologic QDR-2000 dual-energy X-ray absorptiometer was used to make bone mineral density measurements (anterioposterior direction) at lumbar spine and hip with subjects in a supine position. Bone mineral densities were taken at baseline and at 6 and 12 months. Vertebral measures were from L1 to L4. Proximal femur measures were from femoral neck, trochanter, and Ward’s triangle. Each measurement was expressed as both an absolute bone mineral density value and as a z-transformed value (age corrected, based on Hologic norms). Dual-energy X-ray absorptiometer measurements were reviewed by a radiologist to ensure technical quality and that the measures did not include areas of vascular calcification or degenerative arthritis.
Whole blood and urine samples for measures of hormones, cotinine, and peripheral markers of bone metabolism were collected in the morning at baseline and at 3, 6, and 12 months. The markers included plasma osteocalcin and bone-specific alkaline phosphatase (markers of osteoblastic activity) and urinary N-telopeptide (marker of osteoclastic activity). Given concerns about health risks associated with extreme prolactin levels, laboratory reports were monitored. In two cases, patients had prolactin levels >200 ng/ml. In both cases, the dose was reduced, which resolved the safety concern. However, this had the effect of reducing the group mean prolactin levels at the 6-month and 12-month intervals.
For statistical analysis, bone mineral density and marker values served as separate dependent variables in a between-group (high versus low) repeated-measures (time) analysis of variance (ANOVA) model (SPSS for Windows version 9.0, Chicago). Alpha level was set at p<0.05. Analysis of covariance (ANCOVA) was performed to explore potential mediating influences of age, age at symptom onset, duration of illness, body mass index, and degree of smoking (as measured by cotinine levels).
Results
High and low prolactin subgroups were established at baseline and the differential prolactin levels were maintained over time. Between-group ANOVA findings from the prolactin levels showed a significant group effect (F=17.7, df=1, 12, p<0.001); effects of time and the group-by-time interaction were nonsignificant. Mean values for the high prolactin group were greater than those for the low prolactin group (p<0.01 at all time points). Subgroup membership remained constant.
The between-group repeated-measures ANOVA was used to compare bone mineral density (z-transformed, age-corrected values) of the high and low prolactin groups at baseline, 6, and 12 months. Sites were lumbar spine (mean values from L1 to L4) and hip (femoral head) opposite of the dominant hand (i.e., right hand dominant, left hip). There were no statistically significant differences between the high and low prolactin groups on any bone mineral density measures. Exploratory ANCOVAs did not modify these nonsignificant findings.
Individual ANOVAs were performed on the three markers. Results for both plasma osteocalcin and urinary N-telopeptide yielded nonsignificant main effects for group and time. However, the group-by-time interaction was statistically significant in both cases (plasma osteocalcin interaction: F=5.45, df=3, 36, p<0.003; urinary N-telopeptide interaction: F=4.97, df=3, 36, p<0.01). As shown in Figure 1, high prolactin was associated with increased activity for both peripheral markers, whereas low prolactin levels were associated with decreased activity over time. ANCOVAs did not modify this result.
The ANOVA of bone-specific alkaline phosphatase failed to yield any statistically significant effects. ANCOVAs were nonsignificant.
Pearson correlations (df=12) between plasma osteocalcin and urinary N-telopeptide values were nonsignificant at baseline (r=0.02) but were significant over time (3 months: r=0.62 [p<0.02]; 6 months: r=0.63 [p<0.02]; 12 months: r=0.80 [p<0.001]).
Discussion
To our knowledge, this is the first study to examine prospectively the impact of sustained neuroleptic-induced hyperprolactinemia on bone mineral density and osteoblastic/osteoclastic processes in schizophrenic patients. Elevated serum prolactin levels were not associated with significant changes in vertebral or femur bone mineral densities over the 12-month period. Perhaps bone mineral density is affected only at much higher prolactin levels or over longer time periods.
In contrast, results from the markers suggest that hyperprolactinemia has a direct impact on rate of bone metabolism. The process of bone formation and resorption is primarily regulated to compensate for change, such that increases in bone formation stimulate a secondary increase in resorption (a process called coupling), and the degree to which bone mass is maintained depends on the close coordination of these compensatory processes. In this study, elevated prolactin was associated with increased activity in both formation and resorption, and the degree of coupling increased over time. This is in line with previous findings from Fujimaki and colleagues (6), who found that the degree of coupling of bone formation and absorption increased in a nonpsychiatric study group of 21 hyperprolactinemic women. Perhaps higher levels of serum prolactin over a longer time period are necessary before osteoblastic and osteoclastic activity become uncoupled, leading to bone mineral density loss.
Several factors limit the generalizability of this study. Gender was controlled, as was age to a degree. It was not possible to match these groups for other risk factors such as prior neuroleptic history, which is a particularly critical variable. Other important risk factors not considered were prior weight, history of smoking, level of physical activity, and nutritional deficiencies. Given the multifactorial risks culminating in osteoporosis, there were numerous sources of uncontrolled bias that would be difficult to eliminate. The repeated-measures ANOVA model (within-subject comparisons) helped to minimize some of the unknown biases. Nevertheless, these results must be viewed with caution given the small sample size and inherent difficulty of matching patient histories for risk factors. A larger study group will provide a much better estimate of the robustness of these preliminary results.
Presented in part at the 57th annual meeting of the Society of Biological Psychiatry, Philadelphia, May 18–22, 2002. Received Oct. 29, 2002; revision received Feb. 6, 2003; accepted Feb. 27, 2003. From the Arthur P. Noyes Research Foundation; the Department of Psychiatry, Drexel University College of Medicine, Philadelphia; the Department of Psychiatry, University of Pennsylvania, Philadelphia; and the Department of Professional Psychology, Chestnut Hill College, Philadelphia. Address reprint requests to Dr. Josiassen, The Arthur P. Noyes Research Foundation, 1001 Sterigere St., Norristown, PA 19401; [email protected] (e-mail). Supported in part by a grant from Eli Lilly and Company with additional support from The Arthur P. Noyes Research Foundation. The authors thank Rita A. Shaughnessy, M.D., Ph.D., Meera Jessani, M.D., Ronald Friedman, M.D., and Erika Csomor for their comments on the manuscript and Rachel Josiassen for preparing the manuscript.
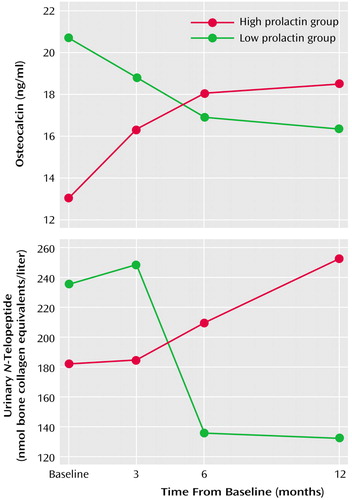
Figure 1. Relation of Bone Metabolism to Serum Prolactin Level in 14 Women With Schizophrenia Treated With Risperidone or Olanzapine Monotherapy Over 12 Monthsa
aPatients placed in high or low prolactin group according to median split. Significant group-by-time interactions were seen for these markers of bone formation (plasma osteocalcin: F=5.45, df=3, 36, p<0.003) and bone resorption (urinary N-telopeptide: F=4.97, df=3, 36, p<0.01).
1. Meltzer HY, Busch DA, Fang VS: Serum neuroleptic and prolactin levels in schizophrenic patients and clinical response. Psychiatry Res 1983; 9:271–283Crossref, Medline, Google Scholar
2. Klibanski A, Neer R, Beitins IZ, Ridgway EC, Zervas NT, McArthur JW: Decreased bone density in hyperprolactinemic women. N Engl J Med 1980; 303:1511–1514Crossref, Medline, Google Scholar
3. Greenspan SL, Neer RM, Ridgway EC, Klibanski A: Osteoporosis in men with hyperprolactinemic hypogonadism. Ann Intern Med 1986; 104:777–782Crossref, Medline, Google Scholar
4. Abraham G, Friedman RH, Verghese C, DeLeon J: Osteoporosis and schizophrenia: can we limit known risk factors? Biol Psychiatry 1995; 38:131–132Crossref, Medline, Google Scholar
5. Halbreich U, Palter S: Accelerated osteoporosis in psychiatric patients: possible pathophysiological processes. Schizophr Bull 1996; 22:447–464Crossref, Medline, Google Scholar
6. Fujimaki T, Kurabayashi T, Ootani T, Yamamoto Y, Yasuda M, Tanaka K: A study on bone metabolism in women with hyperprolactinemia. Nippon Sanka Fujinka Gakkai Zasshi 1994; 46:423–428Medline, Google Scholar



