Does Cognitive Recovery After Treatment of Poststroke Depression Last? A 2-Year Follow-Up of Cognitive Function Associated With Poststroke Depression
Abstract
OBJECTIVE: Cognitive impairment is common after stroke and may be caused by poststroke depression. Remission of poststroke major depression after treatment has been associated with improvement in cognitive function. The current study was designed to examine how long that cognitive improvement lasts and to compare depressed patients’ cognitive status with that of nondepressed patients with comparable lesions. METHOD: Seventeen patients with poststroke depression and cognitive impairment who had early and sustained remission of their depression during a double-blind treatment study were compared with 42 nondepressed stroke patients who remained nondepressed throughout the follow-up. Mood and cognitive function were followed-up over 2 years with the Hamilton Depression Rating Scale and Mini-Mental State Examination (MMSE). RESULTS: In the patients with early and sustained remission of depression, there was rapid improvement of cognitive function, which was maintained over 2 years. Their initial MMSE score of 23.3 (SD=4.2) improved to 26.6 (SD=3.5) at 3 months and was 26.1 (SD=3.6) at 2 years. The nondepressed patients showed essentially no change in cognitive function over 2 years (initial MMSE score: mean=26.3, SD=3.1; score at 2-year follow-up: mean=25.7, SD=4.1). CONCLUSIONS: Cognitive function, once improved after remission of poststroke depression, is likely to remain stable over the next 2 years in the absence of subsequent reinjury to the central nervous system. Cognitive impairment due to poststroke depression is reversible and can be quantified separately from cognitive impairment on the basis of the location and extent of ischemic brain damage.
Cognitive impairment is a common complication after stroke, with a prevalence varying from 13.6% to 35.2% (1–7). The impairment may range from a minimal deficit in memory (8) to a transient disturbance of orientation (9) to an irreversible dementia (3, 5, 10). Figures about the incidence of cognitive impairment after stroke have varied on the basis of the population studied, criteria used to determine cognitive impairment or dementia, type of neuropsychological testing used, time after stroke at which cognitive status was assessed, and whether the patients were depressed.
The role of depression in causing a reversible dementia or pseudodementia has been known for a long time (11–15). Among depressed stroke patients with cognitive impairment, the reversal of cognitive impairment with improved mood following successful treatment of depression has been demonstrated in several studies (16–18). However, little is known of the long-term progression of cognitive changes among stroke patients after this initial reversal of cognitive impairment with successful treatment of depression. Another interesting question is whether this element of reversible cognitive impairment due to depression can be quantified separately from cognitive impairment due to other aspects of stroke.
In this investigation, we studied cognitive changes over 2 years in a group of depressed stroke patients with initial cognitive impairment who were treated for depression in a double-blind study. We hypothesized that for patients with early and sustained remission of depression, cognition would rapidly improve with depression and remain improved over the 2 years of follow-up.
Method
Subjects
Two groups of patients studied at different times were included in this study. The first group consisted of 104 patients who were recruited between June 1991 and June 1997. The vast majority of patients enrolled in this study were inpatients at the Younkers Rehabilitation Center of the Iowa Methodist Medical Center in Des Moines, Iowa (N=89). A small number were recruited from the University of Iowa Hospitals and Clinics in Iowa City (N=1), the VA Medical Center in Iowa City (N=2), and the Raul Carrea Institute of Neurological Research in Buenos Aires (N=12). Patients between the ages of 18 and 85 years who had suffered an acute stroke within the past 6 months and who agreed to participate in a double-blind treatment study of depression were included in the study. The second group consisted of 147 patients who were recruited between January 1980 and May 1990. All of the patients enrolled in this study were from patients consecutively admitted to the University of Maryland Hospital, Baltimore, with a diagnosis of either acute intracerebral hemorrhage or cerebral infarction; the study included 2 years of longitudinal follow-up. The exclusion criteria were 1) any significant medical condition that was life-threatening or would interfere with recovery from stroke, 2) severe comprehension deficit due to decreased consciousness, dementia, or aphasia, 3) history of previous head injury, and 4) history of any previous brain disease other than stroke.
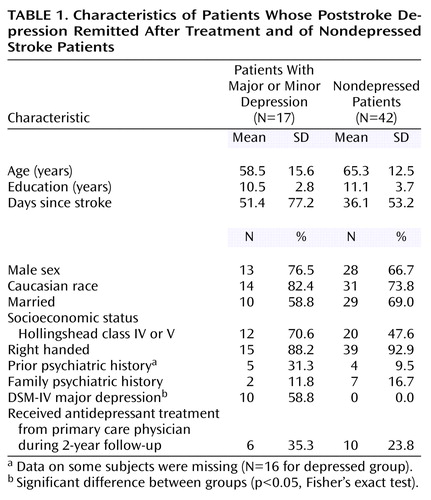
After the procedure was fully explained and signed informed consent was obtained, patients were evaluated with a version of the Present State Examination (PSE) (19), a semistructured interview modified to assess DSM-IV diagnostic criteria. Patients were diagnosed as having depression due to stroke—either major depression (DSM-IV) or minor depression (based on DSM-IV research diagnostic criteria)—on the basis of symptoms elicited by the PSE. The severity of depression was measured by using the 17-item Hamilton Depression Rating Scale (20). The Mini-Mental State Examination (MMSE) (21, 22) was used to assess cognitive impairment. Scores on the MMSE range from 0 to 30, with lower scores indicating greater cognitive impairment. It is an 11-item instrument that has been found to be reliable and valid in assessing cognitive function in a variety of conditions, including dementia and brain injury (23). The neurological examination was conducted by a neurologist using the NIH Stroke Scale (24). The use of these instruments with stroke patients has been validated in other studies (25, 26). The patients were followed up and assessed at 3, 6, 9, 12, 18, and 24 months (at 3, 6, 12, and 24 months for 20 patients from Baltimore). Remission of depression was defined as having a 50% or greater reduction in the initial score on the Hamilton depression scale and no longer meeting the diagnostic criteria for major or minor depression.
The 104 Iowa and Buenos Aires patients in the initial treatment study of poststroke depression have been described elsewhere (27). For the current study, the 12 patients from Argentina, who were not followed-up after the initial treatment trial, were excluded. Among the remaining patients, 47 were depressed and completed the 12-week double-blind treatment trial. Of these 47 depressed patients, during the 21-month naturalistic follow-up period three dropped out in the first 3 months because of side effects of medication. Two died within the first 6 months, and three deteriorated medically. Eleven others withdrew from the study within the first 6 months, refusing treatment or further participation. Of the remaining 28 depressed patients, 12 had an early and sustained remission of their depression, were assessed at 3, 6, 9, 12, 18, and 24 months, had no recurrence of stroke or other serious physical illness, and were selected for this study. A comparison group of 27 nondepressed patients, who were also followed up regularly for 2 years, were selected on the basis of comparable age, race, marital status, education, type of stroke, and side of lesion. The treatments received by the 12 depressed patients included nortriptyline for 3 months at doses titrated up to 100 mg/day (N=3), fluoxetine for 3 months at doses titrated up to 40 mg/day (N=5), and placebo (N=4). Among the 27 nondepressed patients, 10 were treated with the same doses of nortriptyline, five were given the same doses of fluoxetine, and 12 were given placebo.
The 147 Baltimore patients in the longitudinal study of poststroke depression have also been described elsewhere (28). Among them, 63 patients were diagnosed as having depression at enrollment. Of the 63 depressed patients, only five had an early and sustained remission of their depression and were assessed up to 24 months with no severe complications or recurrence of stroke. A comparable group of 15 nondepressed patients were also selected on the basis of the same background and lesion factors as for the other patient groups. Since it was a naturalistic study, no patients were treated actively at enrollment.
Computerized Tomography
Computerized tomography was performed by the hospitals that provided short-term care for the patients, and scans were obtained from them for the purpose of this study. Scan readings were carried out by a neurologist who was blind to any of the psychiatric assessments. The size of the lesion was calculated as the percentage of total brain volume by using the ratio of the largest cross-sectional area of the lesion to the area of the brain slice that included the body of the lateral ventricles (28). Lesions were localized by the brain structures involved and classified as thromboembolic or hemorrhagic.
Statistical Analysis
Intergroup comparisons were carried out by using means, standard deviations, analysis of variance (ANOVA), and Spearman’s correlation coefficient (rs). Frequency distributions were compared by using the chi-square test or Fisher’s exact test, if the cell sizes were too small. All tests were two-tailed.
Results
Patient Characteristics
The background characteristics, depression diagnoses, and antidepressant treatment after the initial treatment trial or during the naturalistic longitudinal follow-up are shown on Table 1. Other than depression diagnosis, there were no significant differences between the depressed and nondepressed patients.
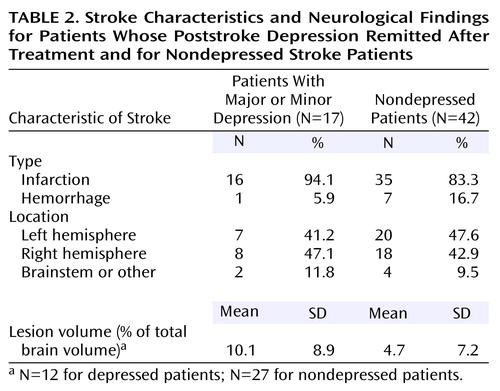
The radiological findings are shown in Table 2. The mean volume of the stroke lesion (expressed as percentage of total brain volume) for the depressed patients was more than twice the volume for the nondepressed patients. There were no significant differences between groups in lesion volume, type, or location.
Longitudinal Findings
The initial mean score on the Hamilton depression scale for the depressed patients, 15.1 (SD=1.2), was significantly higher than the initial score for the nondepressed patients (mean=5.6, SD=4.7) (t=6.78, df=57, p<0.01). There was a rapid improvement in mood over the first 3 months for the depressed patients, with the mean Hamilton depression scale score falling to 5.1 (SD=4.6) at 3 months and 4.9 (SD=4.7) at 6 months (Figure 1). The depressed patients remained in remission from 3 months to 2 years. For the nondepressed patients, there was not much change around the baseline Hamilton depression scale score throughout the 2 years. Repeated-measures ANOVA showed a significant group-by-time interaction, with the depressed group improving more than the nondepressed group over the first 3 months.
The initial mean MMSE score for the depressed patients was 23.3 (SD=4.2), which was significantly worse than the initial mean score of 26.3 (SD=3.1) for the nondepressed group (t=–3.00, df=57, p<0.01). The MMSE scores for the depressed patients improved significantly by the 3-month follow-up, to a mean of 26.6 (SD=3.5) (Figure 1). After this initial rise, the MMSE score for the depressed patients remained fairly steady, around an average of 26.2 points, for the next 2 years (final score: mean=26.1, SD=3.6). These findings demonstrate the sustained improvement in cognitive function after depression is alleviated. For the nondepressed patients, the mean MMSE score showed no significant changes during the 2-year follow-up (final score: mean=25.7, SD=4.1). Repeated-measures ANOVA of the MMSE scores showed a significant group-by-time interaction, with the depressed patients showing significantly more improvement in MMSE scores over the first 3 months than the nondepressed patients. MMSE scores improved rapidly in association with the declining Hamilton depression scale scores (Figure 1). The change in MMSE scores over the first 3 months was significantly inversely correlated with the improvement in Hamilton depression scale scores (rs=–0.31, N=59, p=0.02).
Treated Depression Versus Spontaneous Remission
Because some of our patients had spontaneous remissions of depression, we analyzed separately all of the 39 patients in the original treatment study (i.e., the Iowa stroke study [N=39, treatment study], which does not include the patients in the Baltimore stroke study [N=20, naturalistic study]) who received active treatment or placebo in the double-blind protocol for 3 months. Repeated-measures ANOVA demonstrated a significant time-by-treatment interaction (F=15.39, df=1, 37, p<0.01); the initially depressed patients who received active treatment (N=12) had significantly greater improvement in MMSE scores over the first 3 months than did the nondepressed patients (N=27).
Logistic Regression Analysis
Because there were several differences between the depressed and nondepressed groups, we examined the change in MMSE scores after controlling for variables that significantly differed between the two treatment groups. A logistic regression analysis of improvement in MMSE scores examining the effects of lesion volume, presence of motor deficits, and occurrence of 50% or greater improvement in Hamilton depression score showed a significant and independent effect of depression remission on improvement in MMSE score during the acute phase (i.e., the 6 months following the stroke) (Wald statistic=4.31, df=1, p=0.04) and no significant effect for either lesion volume (Wald statistic=3.06, df=1, p=0.08) or motor deficit (Wald statistic=1.53, df=1, p=0.22). These findings are consistent with the hypothesis that the remission of depression was related to improvement of cognitive function.
Discussion
This study demonstrated a rapid improvement in cognitive function in patients with poststroke depression who showed an early and sustained remission of their depression. In addition, the improvement among patients with remitted depression was maintained over 2 years. To our knowledge, this is the first time that treatment or remission of poststroke depression has been shown to produce a cognitive improvement that lasts more than 2 years if no subsequent illness occurs.
Before the findings and their implications are discussed further, the limitations in this study need to be acknowledged. First, the MMSE is a relatively brief examination that is language dominated. A more detailed neuropsychological battery would have allowed a fuller examination of how the spectrum of cognitive functions and their longitudinal progression was affected by depression. Another limitation is the attrition of patients, mostly within the first 6 months. The patients in the study, who were regularly followed up through 2 years, may have been highly compliant and thus may not be representative of all stroke patients. A third limitation is that administering the MMSE every 3 months may have led to a practice effect. However, the leveling off of the MMSE score in the depressed patients and the lack of any improvement in the nondepressed patients suggest that this is not so. A final limitation, which is inherent not only in this study but also in most studies on poststroke cognitive impairment, is that the prestroke cognitive status of the subjects was not known and quantified. Most studies have identified and enrolled subjects after the onset of stroke and relied on retrospective approximation of the prestroke cognitive status from interviews with the subjects or close relatives. Without a prestroke neuropsychological assessment, it is difficult to ascertain how and to what extent cognitive functions were affected by stroke. Because of the similarities in age and education, however, overall group differences in premorbid cognitive function would not be expected.
As far as we are aware, one of the few, perhaps the only, study that was able to compare pre- and poststroke cognitive assessments is the Framingham study (29). In this prospective observational study, participants were followed up biennially, and MMSEs were carried out. For participants who subsequently developed stroke, another MMSE was carried out 6 months after their illness. Participants in this study were generally older; the mean age for men was 76.0 years (SD=0.7), and for women it was 81.0 years (SD=0.8). The stroke patients had a prestroke mean MMSE score of 27.28 (SD=0.34) and a poststroke score of 23.57 (SD=0.92), while nonstroke control subjects had mean scores of 28.08 (SD=0.21) and 28.31 (SD=0.25), respectively. The change in MMSE score differed significantly between the stroke patients and control subjects. After the stroke, 31.1% of the patients had an abnormal MMSE score (defined as lower than 24). The importance of the Framingham study is that it demonstrates that about 70% of the stroke patients, although not demented or having an abnormal MMSE score after the stroke, nonetheless suffered some degree of cognitive decline after the stroke, which was quantified as 3.71 points of mean decline on the MMSE.
On the basis of the Framingham data, the initial mean MMSE score of 26.3 (SD=3.1) for the nondepressed patients in our study almost certainly represents a decline from their prestroke cognitive ability. This is further corroborated by comparison of their MMSE scores with population-based norms for age and educational level (30); the mean score for people ages 65–69 years with 9–12 years of education is 28 (SD=1.4). The depressed patients had an initial mean MMSE score of 23.3 (SD=4.2), which was 3.0 points lower than that for the nondepressed patients. After remission of the depression, the MMSE scores for the depressed and nondepressed patients at 6 months were almost identical (mean=26.5, SD=3.1, versus mean=27.1, SD=3.1), but they were still lower than the population-based mean norm of 28 (SD=1.4) for people ages 65–69 years who have 9–12 years of education (30). Thus, the effects of brain infarction and depression on cognitive function appear to be additive.
For the patients in this study, the degree of cognitive impairment due to depression was 3.3 points on the MMSE (difference between the initial score of 23.3 and the mean score of 26.6 after remission of depression). During the longitudinal follow-up, the nondepressed patients showed no significant change in MMSE scores over 2 years. This finding suggests that the cognitive deterioration due to ischemic brain injury does not improve between 1 month and 24 months poststroke.
The determinants of cognitive impairment due to ischemic brain damage have been described in several studies. The Framingham study showed a correlation between poststroke cognitive decline and large left-sided strokes (29). Tatemichi et al. (7), by examining a broad range of neuropsychological deficits independently of a diagnosis of dementia, found cognitive impairment (described as failure on any four items in a battery of tests) in 35.2% of stroke patients and an association of cognitive impairment with major cortical syndromes and infarctions in the territories of the left anterior and posterior cerebral arteries. Moderately severe cognitive impairment, diminished alertness in the acute stroke stage, temporal lobe infarcts, multiple infarcts, and pronounced ventricular enlargement were found by Schmidt et al. (31) to result in high risk for long-term intellectual dysfunction. Our group (32) has found that long-term cognitive impairment may be associated with subcortical caudate strokes. The determinants of poststroke dementia have been found by Censori et al. (2) to be diabetes mellitus, atrial fibrillation, aphasia, large middle cerebral artery infarcts, and frontal lobe lesions. Desmond et al. (3) found the determinants to include 1) stroke characteristics, such as the severity and location of the stroke, especially with more severe left hemispheric infarcts in the territories of the anterior and posterior cerebral arteries, 2) vascular risk factors of diabetes mellitus and previous stroke, and 3) host characteristics, such as greater age, fewer years of education, and nonwhite race/ethnicity.
Poststroke cognitive impairment related to major depression has been studied by our group for several years (17, 18, 26, 28, 33, 34). In an earlier study (35), we compared patients with major depression and nondepressed patients matched for lesion size and location and found a difference of 5.8 points in MMSE score between depressed patients (mean=16.6, SD=7.8) and nondepressed patients (mean=22.4, SD=6.6) (p=0.02). In addition, we found that depression was associated with cognitive impairment only for the first year after the stroke and that the effect of depression on cognitive function was strongest during the acute poststroke period (34).
We have also demonstrated (17) improved cognition among patients whose depression remitted after treatment with nortriptyline or placebo. This suggested that the improvement in cognitive function was due to the mechanism of remission of major depression and was not due to a direct effect of nortriptyline on cognition. This conclusion is consistent with our finding in the present study that 10 nondepressed patients given nortriptyline showed no significant improvement in MMSE score.
Assessment of the use of antidepressant medication after the first 12 weeks of the treatment trial, when patients were returned to the care of their primary care physicians, revealed no difference between depressed and nondepressed patients in the frequency or duration of antidepressant use. This suggested that the sustained improvement in the cognitive function of the depressed patients was not due to prolonged use of antidepressants but was associated with sustained remission of depression.
Some studies on elderly populations with depression or stroke have shown long-term cognitive deterioration. Alexopoulos et al. (36, 37), who followed up on elderly patients (average age, 73 years) with reversible depressive dementia without stroke, found that 43% developed an irreversible dementia about 33 months later. On the basis of this finding, the authors proposed that patients who have late-onset depression with reversible dementia may include some who have a preexisting early-stage dementia (36, 37). Moroney et al. (38) followed up on nondemented stroke patients (average age, 70 years) for 53 months and found that hypoxic-ischemic events after the stroke, such as seizures, cardiac arrhythmias, and pneumonia, resulted in a higher risk for subsequent dementia.
The absence of deterioration of cognitive function in our patients over 2 years in this study may be due to the fact that they were younger than patients in other studies and that the follow-up was shorter. Further cognitive deterioration after an initial stroke may be related to age, duration since stroke, or subsequent reinjury to the central nervous system, such as another stroke, hypoxic-ischemic events, or the onset of neurodegenerative disorders.
In summary, poststroke cognitive dysfunction associated with depression, once improved, is likely to remain stable over the next 2 years, unless there is subsequent reinjury to the central nervous system. Hence, there is a need for early detection and treatment of poststroke depression in order to maximize cognitive function.
 |
 |
Received March 19, 2001; revision received June 12, 2002; accepted Jan. 22, 2003. From the Department of Psychiatry, University of Iowa College of Medicine. Address reprint requests to Dr. Robinson, Department of Psychiatry, University of Iowa College of Medicine, 2887 JPP, 200 Hawkins Dr., Iowa City, IA 52242; [email protected] (e-mail). Supported in part by NIMH grants MH-40355, MH-52879, and MH-53592. The authors thank Charles F. Denhart, M.D., Toru Nishikawa, M.D., Ph.D., Eisuke Matsushima, M.D., Ph.D., the Younkers Rehabilitation Center of the Iowa Methodist Medical Center in Des Moines, Iowa, and the neurology departments of the University of Iowa Hospitals and the VA Medical Center in Iowa City for allowing assessment of patients for inclusion in the study.
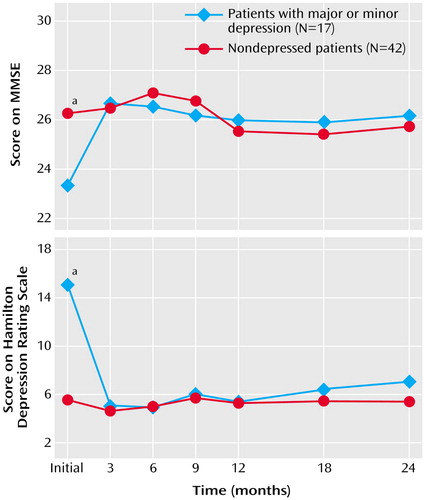
Figure 1. Scores Over 2 Years on the Mini-Mental State Examination (MMSE) and Hamilton Depression Rating Scale for Patients Whose Poststroke Depression Remitted After Treatment and for Nondepressed Stroke Patientsa
aRepeated-measures ANOVAs showed significant group-by-time interactions for scores on both the MMSE (F=2.46, df=6, 52, p=0.04) and Hamilton depression scale (F=13.99, df=6, 52, p<0.01).
1. Barba R, Martinez-Espinosa S, Rodriguez-Garcia E, Pondal M, Vivancos J, Del Ser T: Poststroke dementia: clinical features and risk factors. Stroke 2000; 31:1494-1501Crossref, Medline, Google Scholar
2. Censori B, Manara O, Agostinis C, Camerlingo M, Casto L, Galavotti B, Partziguian T, Servalli MC, Cesana B, Belloni G, Mamoli A: Dementia after first stroke. Stroke 1996; 27:1205-1210Crossref, Medline, Google Scholar
3. Desmond DW, Moroney JT, Paik MC, Sano M, Mohr JP, Aboumatar S, Tseng CL, Chan S, Williams JB, Remien RH, Hauser WA, Stern Y: Frequency and clinical determinants of dementia after ischemic stroke. Neurology 2000; 54:1124-1131Crossref, Medline, Google Scholar
4. Inzitari D, Di Carlo A, Pracucci G, Lamassa M, Vanni P, Romanelli M, Spolveri S, Adriani P, Meucci I, Landini G, Ghetti A: Incidence and determinants of poststroke dementia as defined by an informant interview method in a hospital-based stroke registry. Stroke 1998; 29:2087-2093Crossref, Medline, Google Scholar
5. Pohjasvaara T, Erkinjuntti T, Ylikoski R, Hietanen M, Vataja R, Kaste M: Clinical determinants of poststroke dementia. Stroke 1998; 29:75-81Crossref, Medline, Google Scholar
6. Tatemichi TK, Desmond DW, Mayeux R, Paik M, Stern Y, Sano M, Remien RH, Williams JB, Mohr JP, Hauser WA: Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology 1992; 42:1185-1193Crossref, Medline, Google Scholar
7. Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E: Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry 1994; 57:202-207Crossref, Medline, Google Scholar
8. Bowler JV, Hadar U, Wade JP: Cognition in stroke. Acta Neurol Scand 1994; 90:424-429Crossref, Medline, Google Scholar
9. Desmond DW, Tatemichi TK, Figueroa M, Gropen TI, Stern Y: Disorientation following stroke: frequency, course, and clinical correlates. J Neurol 1994; 241:585-591Crossref, Medline, Google Scholar
10. Kokmen E, Whisnant JP, O’Fallon WM, Chu CP, Beard CM: Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960-1984). Neurology 1996; 46:154-159Crossref, Medline, Google Scholar
11. Kiloh LG: Pseudo-dementia. Acta Psychiatr Scand 1961; 37:336-351Crossref, Medline, Google Scholar
12. Caine ED: Pseudodementia: current concepts and future directions. Arch Gen Psychiatry 1981; 38:1359-1364Crossref, Medline, Google Scholar
13. Rabins PV: The prevalence of reversible dementia in a psychiatric hospital. Hosp Community Psychiatry 1981; 32:490-492Abstract, Google Scholar
14. Rabins PV, Merchang A, Nestadt G: Criteria for diagnosing reversible dementia caused by depression: validation by 2-year follow-up. Br J Psychiatry 1984; 144:488-492Crossref, Medline, Google Scholar
15. Austin MP, Ross M, Murray C, O’Carroll RE, Ebmeier KP, Goodwin GM: Cognitive function in major depression. J Affect Disord 1992; 25:21-29Crossref, Medline, Google Scholar
16. Gonzalez-Torrecillas JL, Mendlewicz J, Lobo A: Effects of early treatment of post-stroke depression on neuropsychological rehabilitation. Int Psychogeriatr 1995; 7:547-560Crossref, Medline, Google Scholar
17. Kimura M, Robinson RG, Kosier JT: Treatment of cognitive impairment after poststroke depression: a double-blind treatment trial. Stroke 2000; 31:1482-1486Crossref, Medline, Google Scholar
18. Murata Y, Kimura M, Robinson RG: Does cognitive impairment cause post-stroke depression? Am J Geriatr Psychiatry 2000; 8:310-317Crossref, Medline, Google Scholar
19. Wing JK, Cooper JE, Sartorius N: The Measurement and Classification of Psychiatric Symptoms: An Instructional Manual for the PSE and CATEGO Programs. New York, Cambridge University Press, 1974Google Scholar
20. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
21. Folstein MF, Robins LN, Helzer JE: The Mini-Mental State Examination. Arch Gen Psychiatry 1983; 40:812Crossref, Medline, Google Scholar
22. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
23. McHugh PR, Folstein MF: Psychopathology of dementia: implications for neuropathology. Res Publ Assoc Res Nerv Ment Dis 1979; 57:17-30Medline, Google Scholar
24. Kunitz SC, Gross CR, Heyman A, Kase CS, Mohr JP, Price TR, Wolf PA: The Pilot Stroke Data Bank: definition, design, and data. Stroke 1984; 15:740-746Crossref, Medline, Google Scholar
25. Robinson RG, Benson DF: Depression in aphasic patients: frequency, severity and clinical-pathological correlations. Brain Lang 1981; 14:282-291Crossref, Medline, Google Scholar
26. Robinson RG, Starr LB, Kubos KL, Price TR: A two year longitudinal study of post-stroke mood disorders: findings during the initial evaluation. Stroke 1983; 14:736-744Crossref, Medline, Google Scholar
27. Robinson RG, Schultz SK, Castillo C, Kopel T, Kosier JT, Newman RM, Curdue K, Petracca G, Starkstein SE: Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry 2000; 157:351-359Link, Google Scholar
28. Robinson RG, Bolla-Wilson K, Kaplan E, Lipsey JR, Price TR: Depression influences intellectual impairment in stroke patients. Br J Psychiatry 1986; 148:541-547Crossref, Medline, Google Scholar
29. Kase CS, Wolf PA, Kelly-Hayes M, Kannel WB, Beiser A, D’Agostino RB: Intellectual decline after stroke: the Framingham Study. Stroke 1998; 25:805-812Crossref, Google Scholar
30. Crum RM, Anthony JC, Bassett SS, Folstein MF: Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993; 269:2386-2391Crossref, Medline, Google Scholar
31. Schmidt R, Mechtler L, Kinkel PR, Fazekas F, Kinkel WR, Freidl W: Cognitive impairment after acute supratentorial stroke: a 6-month follow-up clinical and computed tomographic study. Eur Arch Psychiatry Clin Neurosci 1993; 243:11-15Crossref, Medline, Google Scholar
32. Bokura H, Robinson RG: Long-term cognitive impairment associated with caudate stroke. Stroke 1997; 28:970-975Crossref, Medline, Google Scholar
33. Bolla-Wilson K, Robinson RG, Starkstein SE, Boston J, Price TR: Lateralization of dementia of depression in stroke patients. Am J Psychiatry 1989; 146:627-634Link, Google Scholar
34. Downhill JE Jr, Robinson RG: Longitudinal assessment of depression and cognitive impairment following stroke. J Nerv Ment Dis 1994; 182:425-431Crossref, Medline, Google Scholar
35. Starkstein SE, Robinson RG, Price TR: Comparison of patients with and without post-stroke major depression matched for size and location of lesion. Arch Gen Psychiatry 1988; 45:247-252Crossref, Medline, Google Scholar
36. Alexopoulos GS, Young RC, Meyers BS: Geriatric depression: age of onset and dementia. Biol Psychiatry 1993; 34:141-145Crossref, Medline, Google Scholar
37. Alexopoulos GS, Meyers BS, Young RC, Mattis S, Kakuma T: The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry 1993; 150:1693-1699Link, Google Scholar
38. Moroney JT, Bagiella E, Desmond DW, Paik MC, Stern Y, Tatemichi TK: Risk factors for incident dementia after stroke: role of hypoxic and ischemic disorders. Stroke 1996; 27:1283-1289Crossref, Medline, Google Scholar



