Placental Passage of Antidepressant Medications
Abstract
OBJECTIVE: This study determined the placental transfer of antidepressants and their metabolites. METHOD: A total of 38 pregnant women taking citalopram, fluoxetine, paroxetine, or sertraline participated. Maternal and umbilical cord blood samples were obtained to determine antidepressant and metabolite concentrations. RESULTS: Antidepressant and metabolite concentrations were detectable in 86.8% of umbilical cord samples. The mean ratios of umbilical cord to maternal serum concentrations ranged from 0.29 to 0.89. The lowest ratios were for sertraline and paroxetine; the highest were for citalopram and fluoxetine. Maternal doses of sertraline and fluoxetine correlated with umbilical cord concentrations of these medications. CONCLUSIONS: Umbilical cord concentrations of antidepressants and their metabolites were almost invariably lower than corresponding maternal concentrations. Maternal doses predicted umbilical concentrations of fluoxetine and sertraline. Mean umbilical cord to maternal serum ratios were significantly lower for sertraline than fluoxetine, suggesting that sertraline may produce less fetal medication exposure than fluoxetine near delivery.
To date, over 2,000 reported prenatal exposures to selective serotonin reuptake inhibitor antidepressants have not identified a risk for major congenital anomalies (1, 2). However, several reports have described perinatal complications, including jitteriness, irritability, and respiratory difficulties after third-trimester exposure to these antidepressants (2–6). Whether these complications resulted from newborns’ exposure to these medications during pregnancy remains obscure.
Investigations that detail the extent of fetal exposure to medications provide a basis for assessing a potential effect of exposure (e.g., higher concentrations in umbilical cord blood associated with complications) (7). The current investigation sought to extend previous preliminary data (7) by examining the extent of placental passage of antidepressants when these medications are taken in late pregnancy.
Method
A total of 38 pregnant women who came to the Pregnancy and Postpartum Mood Disorders Program at the University of California at Los Angeles (UCLA) were included in the study. All participants were nonsmokers and in good health, with a mean age of 35 years (SD=4). All women were taking antidepressants for the treatment of depression and were taking the medications for a minimum of five half-lives of elimination for each compound studied before delivery. Written informed consent was obtained for collection of serum samples.
Immediately after each birth, a blood sample was collected from the umbilical vein before delivery of the placenta. A maternal serum sample was also obtained. The blood samples were collected in red-top tubes placed in diagnostic specimen containers and mailed by overnight delivery to the UCLA clinical laboratory. Upon arrival at the laboratory, the blood was centrifuged at 950 g for 10 minutes, and the serum was removed and transferred in 1-ml aliquots to sterile polypropylene tubes. The tubes were coded and stored at –20°C until assay.
The method for serum assay has been detailed in previous reports (8). Briefly, the concentration of antidepressants and metabolites in serum was described by means of an isocratic high-performance liquid chromatography separation followed by ultraviolet detection. The assays had a low limit of sensitivity of 1 ng/ml (fluoxetine, norfluoxetine, paroxetine, sertraline, and desmethylsertraline) or 10 ng/ml (citalopram and desmethylcitalopram).
The degree of placental passage was calculated as the ratio of medication concentration of umbilical cord to maternal serum. A ratio of <1.0 would indicate incomplete placental passage. Consistent with our previous reports, concentrations that were below the limit of detection were converted to the limit of detection (e.g., <1 ng/ml=1 ng/ml) for data analysis, thereby erring on the side of overestimation.
Statistical analyses were performed by using nonparametric (Spearman’s) correlations since the distributions of the variables were not normal; t tests were used for comparisons of means.
Results
A total of 38 umbilical cord and 37 maternal serum samples were obtained. Maternal serum samples were obtained within 30 minutes of delivery in 33 cases and within 2–6 hours in five cases.
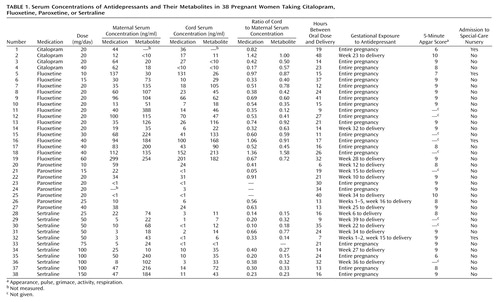
Antidepressant and/or metabolite concentrations were detectable in 33 (86.8%) of 38 umbilical cord serum samples. Umbilical cord and maternal serum concentrations of medication are shown in Table 1. Mean ratios of cord to maternal serum concentrations of antidepressants and metabolites ranged from 0.29 to 0.89, with sertraline producing the lowest ratio and citalopram producing the highest. Mean ratios of cord to maternal serum concentrations were significantly lower for sertraline than for fluoxetine (t=3.35, df=23, p=0.003) and for desmethylsertraline than for norfluoxetine (t=3.04, df=23, p=0.006).
Maternal dose of sertraline correlated significantly with cord serum concentration of sertraline (r=0.71, df=9, p=0.01) and cord serum concentration of desmethylsertraline (r=0.74, df=9, p=0.01). Maternal fluoxetine dose showed a significant relationship with cord serum concentration of norfluoxetine (r=0.80, df=13, p=0.0007) and was nearly significant with serum cord concentration of fluoxetine (r=0.66, df=13, p=0.06). No relationship emerged between maternal dose and cord serum concentration of citalopram (r=–0.87, df=1, p=0.33) or cord serum concentration of paroxetine (r=0.20, df=6, p=0.64).
Neonatal outcomes were obtained from a review of obstetric and pediatric records. All infants were born at 37–41 weeks of gestation and were of normal birth weight. Adverse outcomes occurred for five of 38 infants and were defined broadly to include 5-minute Apgar scores of <7 and special-care nursery admission for any length of time (Table 1). These five infants did not have the highest serum umbilical cord concentrations of medication.
Four infants required special-care nursery admission after delivery. One infant (number 1) was admitted because of hypotonia and apneic episodes. This infant had been exposed throughout pregnancy to clonazepam, a medication that has been linked to neonatal apnea and hypotonia when used prenatally (9). Another infant (number 32) was diagnosed with Hirschsprung’s disease and was admitted to the special-care nursery for surgical correction. Two infants (numbers 5 and 16) required admission to the special-care nursery for respiratory distress attributed to tight nuchal cords.
One infant (number 35) had meconium-stained fluid, a tight nuchal cord, and a low 5-minute Apgar score but did not require admission to the special-care nursery.
Discussion
This study examined umbilical cord serum concentrations of medication and neonatal outcomes after maternal use of antidepressants near term. Umbilical cord serum samples provide a mechanism to examine fetal blood (10) and represent the least invasive estimate of fetal exposure to prenatal medications.
The study found that umbilical cord serum concentrations of antidepressants were almost invariably lower than maternal serum concentrations. Mean ratios of cord to maternal serum concentrations of antidepressants and their metabolites ranged from 0.29 to 0.89. Sertraline produced the lowest ratio, followed by paroxetine. Citalopram produced the highest ratio, followed closely by fluoxetine. Mean ratios of cord to maternal serum concentrations were significantly lower for sertraline than for fluoxetine, suggesting that sertraline may produce less fetal medication exposure than fluoxetine.
Variation in protein binding may explain differences in the ratios for cord to maternal serum concentrations (11): sertraline is the most protein-bound of the four medications, while citalopram is the least (12). Medication half-life, which is the longest for fluoxetine (12), may also have influenced these ratios.
A relatively short medication half-life has been implicated in neonatal withdrawal symptoms after third-trimester exposure (3, 5). In this study, symptoms suggesting withdrawal in the infants were not observed after prenatal exposure to either the long- or short-acting antidepressants. Of note, signs of medication withdrawal in newborns remain to be better defined and distinguished from complications resulting from other causes.
Maternal doses of fluoxetine and sertraline correlated highly with serum cord concentrations of these medications. However, while higher doses of fluoxetine and sertraline resulted in higher cord serum concentrations, the relationship was not 1:1; i.e., a doubling of the dose did not double the fetal exposure. These data suggest that an increase in maternal medication dose during pregnancy will not necessarily be accompanied by a comparable increase in fetal exposure.
None of the infants in this study was born preterm or of low birth weight. This finding contrasts with the national rates of 7.6% and 11.6%, respectively, for these complications (13) but is not surprising since the mothers in this study—healthy nonsmoking middle-class women—generally represent a group at low risk for neonatal complications.
The greater concentrations of metabolite relative to parent compound in the cord serum samples suggest that the fetus is able to metabolize and eliminate the medications—at least to some extent. As fetal drug clearance at term approximates only one-third that of adults (14), it is encouraging to know that maternal use of antidepressants in late pregnancy does not lead to accumulation of medication in fetal circulation.
 |
Received April 10, 2002; revision received Sept. 13, 2002; accepted Oct. 17, 2002. From UCLA Neuropsychiatric Institute and Hospital; and Emory University School of Medicine, Atlanta. Address reprint requests to Dr. Hendrick, Mood Disorders Research Program, UCLA Neuropsychiatric Institute and Hospital, 300 Medical Plaza, Suite 2345, Los Angeles, CA 90095; [email protected] (e-mail). Supported by NIMH grant MH-01451.
1. Ericson A, Kullen B, Wilholm BE: Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol 1999; 55:503-508Crossref, Medline, Google Scholar
2. Chambers CD, Anderson PO, Thomas RG, Felix RJ, Johnson KA, Jones KL: Weight gain in infants breastfed by mothers who take fluoxetine. Pediatrics 1999; 104:e61Google Scholar
3. Nordeng H, Lindemann R, Perminov KV, Reikvam A: Neonatal withdrawal syndrome after in utero exposure to selective serotonin reuptake inhibitors. Acta Paediatr 2001; 90:288-291Crossref, Medline, Google Scholar
4. Cohen LS, Heller VL, Bailey JW, Grush L, Ablon JS, Bouffard SM: Birth outcomes following prenatal exposure to fluoxetine. Biol Psychiatry 2000; 48:996-1000Crossref, Medline, Google Scholar
5. Dahl ML, Olhager E, Ahiner J: Paroxetine withdrawal syndrome in a neonate. Br J Psychiatry 1997; 171:391-392Crossref, Medline, Google Scholar
6. Kent LSW, Laidlaw JDD: Suspected congenital sertraline dependence (letter). Br J Psychiatry 1995; 167:412-413Crossref, Medline, Google Scholar
7. Stowe ZN, Llewellyn AM, Strader JR, Kilts CD, Ritchie JC, Nemeroff CB: Placental Passage of Antidepressants, in 2000 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1997, number 192Google Scholar
8. Hendrick V, Stowe ZN, Altshuler LL, Mintz J, Hwang S, Hostetter A, Suri R, Leight K, Fukuchi A: Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol Psychiatry 2001; 50:775-782Crossref, Medline, Google Scholar
9. Fisher JB, Edgren BE, Mammel MC, Coleman JM: Neonatal apnea associated with maternal clonazepam therapy: a case report. Obstet Gynecol 1985; 66:345-355Google Scholar
10. Rodeck CH, Nicolini U: Fetal blood sampling. Eur J Obstet Gynecol Reprod Biol 1988; 28:85-89Crossref, Medline, Google Scholar
11. Mucklow JC: The fate of drugs in pregnancy. Clin Obstet Gynecol 1986; 13:161-175Google Scholar
12. DeVane CL: Differential pharmacology of newer antidepressants. J Clin Psychiatry 1998; 59:85-93Crossref, Medline, Google Scholar
13. Martin JA, Hamilton BE, Ventura SJ, Menacker F, Park MM: Births: final data for 2000. Natl Vital Stat Rep 2002; 50:1-101Google Scholar
14. Begg EJ, Atkinson HC, Duffull SB: Prospective evaluation of a model for the prediction of milk: plasma drug concentrations from physicochemical characteristics. Br J Clin Pharmacol 1992; 33:501-505Crossref, Medline, Google Scholar



