Personality Traits and Striatal Dopamine Synthesis Capacity in Healthy Subjects
Abstract
OBJECTIVE: Neuroimaging and genetic studies suggest that individual differences in the brain dopaminergic system contribute to the normal variability of human personality (e.g., social detachment and novelty seeking). The authors studied whether presynaptic dopamine function is also associated with personality traits. METHOD: Presynaptic dopamine synthesis capacity in the brain was measured with positron emission tomography and [18F]fluorodopa in 33 healthy adults, and personality traits were assessed with the Karolinska Scales of Personality. Associations were studied by using a linear regression model controlling for the effects of age and gender on both variables. RESULTS: High scores on two of the anxiety-related personality scales, somatic anxiety and muscular tension, and on one aggressivity-related scale, irritability, were significantly associated with low [18F]fluorodopa uptake in the caudate. No statistically significant associations were observed between [18F]fluorodopa uptake and the detachment scale or scales related to novelty-seeking behavior (impulsiveness and monotony avoidance). CONCLUSIONS: The results suggest a role for the dopaminergic system in the regulation of anxiety in healthy subjects. Together with previous studies, they also indicate differential involvement of various components of the dopaminergic system in normal and pathological personality traits.
The dimensional concept of human personality postulates that seemingly infinite interindividual variability in temperament and character can be explained with a finite number of personality traits, or dimensions. The heuristic value of chosen dimensions is based on the assumption that they describe real, underlying psychological and/or biological phenomena in a meaningful way, rather than being just an arbitrary set of scales without any empirical validity. Undoubtedly, the best-known dimensional hypothesis of personality is Cloninger’s psychobiological model of temperament and character, which in its present form consists of four dimensions of temperament (more biologically determined) and three dimensions of character (shaped later in life and more by learning and environmental factors, according to the hypothesis) (1, 2). Dimensional models can be used to explain not only normal personality but also pathological behavior. In this context, personality disorders can be conceived as manifestations of dimensional extremities (1, 3).
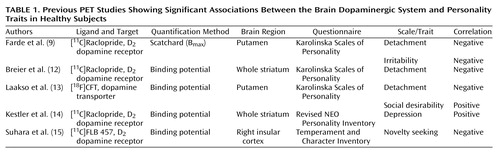
In addition to self-report questionnaires based on Cloninger’s model (Tridimensional Personality Questionnaire, Temperament and Character Inventory), several others have also been used recently in the search for biological markers for different personality traits, e.g., Karolinska Scales of Personality (4), Revised NEO Personality Inventory (5), and Eysenck Personality Questionnaire—Revised (6). Despite different structures and nomenclature in these questionnaires, many traits are shared. For example, the novelty-seeking trait from the Tridimensional Personality Questionnaire and the Temperament and Character Inventory is probably closely related to the monotony avoidance and impulsiveness scales from the Karolinska Scales of Personality, and harm avoidance is probably related to the anxiety-related scales of the Karolinska Scales of Personality (7). In the last several years, the focus of the research on biological factors in personality traits has evolved from the use of peripheral neurobiological markers (such as platelet receptor or enzyme assays [4] or measurement of neurotransmitter metabolites in cerebrospinal fluid [8]) to in vivo brain imaging (9) and studies of genetic polymorphisms, such as certain polymorphisms in exon III of the D4 dopamine receptor gene, which have been examined for the association with Cloninger’s novelty-seeking trait (10, 11). Several positron emission tomographic (PET) brain imaging studies of personality in healthy subjects have concentrated on the dopaminergic system (Table 1 provides a summary). The most consistent finding has been the association between low levels of striatal dopaminergic markers (D2 dopamine receptor and dopamine transporter) and greater social withdrawal and aloofness, as assessed by the detachment scale of the Karolinska Scales of Personality (9, 12, 13). This observation is most probably related to the findings of low levels of in vivo dopamine transporters and D2 receptor binding in the striatum of subjects with social phobia (16, 17), and together they provide robust evidence for the role of the dopaminergic system in human social behavior.
In the present study, presynaptic dopamine synthesis capacity in the striatum of healthy volunteers was measured by using PET and a radiolabeled dopamine precursor, [18F]fluorodopa (which is converted to [18F]fluorodopamine by the enzyme dopa decarboxylase in dopaminergic neurons), and personality traits were assessed with the Karolinska Scales of Personality. This instrument was chosen as a personality questionnaire because of its previous wide use in PET studies of dopamine and personality and its documented validity and stability in Scandinavian subjects (4, 18–20). Previously, associations between personality traits and [18F]fluorodopa uptake have been investigated in patients with Parkinson’s disease (21, 22). However, we know of no published studies on [18F]fluorodopa uptake and personality in healthy subjects, even though these would be desirable to elucidate the role of presynaptic dopamine function in the normal variability of human personality.
Method
Subjects
The study was approved by the Ethical Committee of Turku University and Turku University Central Hospital, and it was conducted according to the Declaration of Helsinki. After complete description of the study to the subjects, a written informed consent statement was obtained. We recruited 33 healthy, nonsmoking volunteers, ages 20–59 years, with no history of mental or neurological illness or substance abuse. The absence of axis I diagnoses was confirmed by a semistructured interview administered by the investigating physician (A.L. or J.K.) before inclusion in the study. None of the participants had a first-degree relative with diagnosed psychiatric disorder. All subjects had normal 1.5-T magnetic resonance imaging (MRI) brain scans. The study group included 22 men and 11 women, and there was no significant age difference between the sexes (men: mean=37 years, SD=11; women: 35 years, SD=11).
PET Procedure
PET scans with [18F]fluorodopa were performed as described previously (23) by using a whole-body PET scanner (ECAT 931/08-12, CTI, Knoxville, Tenn.) with 15 slices. Ninety minutes before the scan, 100 mg of carbidopa, a peripheral dopa decarboxylase inhibitor (Orion Pharma, Espoo, Finland), was given to the subjects. The scans were acquired up to 60 minutes after tracer injection, and uptake was quantified with Patlak analysis by using metabolite-corrected arterial blood samples for the input function and the occipital cortex as a reference region (24). Regions of interest (caudate, putamen, and occipital cortex) were drawn individually for each subject on 1.5-T MRI images resliced according to PET without knowledge of personality scores.
Personality Assessment
Each subject completed the Karolinska Scales of Personality self-report questionnaire (4) on the day of the PET scan. There are 15 different personality scales, and the number of questions for each scale varies from five to 20. Each answer is scored from 1 (does not apply) to 4 (applies completely).
Statistical Methods
Age and gender have been shown to affect scores on the Karolinska Scales of Personality, and T score transformation is usually used to adjust for their effects (resulting in a mean score of 50, SD=10, in all age and gender groups for all scales) (4, 9, 13). However, we have previously demonstrated (25) that age and gender also interact with [18F]fluorodopa uptake in healthy subjects. Therefore, in the primary analysis, a linear regression model was used to control for differential effects of age and gender on unadjusted raw personality scores and [18F]fluorodopa uptake values. The regression model consisted of the response variable, the raw score on the Karolinska Scales of Personality, and the following explanatory variables: [18F]fluorodopa uptake, age, gender, interaction between [18F]fluorodopa uptake and age, interaction between [18F]fluorodopa uptake and gender, and interaction between age and gender. The equation for the linear regression model was as follows: KSP=a+(b×F)+(c×A)+(d×S)+(e×[F×A])+(f×[F×S])+(g×[A×S]), where KSP=raw score on the respective Karolinska Scales of Personality scale, F=[18F]fluorodopa uptake (Ki value) in the studied brain region, A=age, and S=sex. The residuals were assumed to be normally distributed. Since this was an exploratory study, p values below 0.01 were considered statistically significant. In an additional analysis, the results from the linear regression model were subjected to Bonferroni correction, with adjustment for multiple comparisons (two to four brain regions, 15 scales). Bonferroni-adjusted p values below 0.05 were considered statistically significant.
While linear regression analysis was considered essential to control for complex interactions between different variables, we also wanted to calculate Pearson’s correlation coefficients for relationships between [18F]fluorodopa Ki values and T scores on the Karolinska Scales of Personality, as this statistical method has been used in most previous imaging studies (9, 12, 13).
Results
Results from the linear regression analysis are summarized in Table 2. Low [18F]fluorodopa uptake in the caudate in both hemispheres was significantly associated with high somatic anxiety, muscular tension, and irritability scores. Other anxiety-related scales (psychic anxiety, psychasthenia, and inhibition of aggression) also consistently correlated negatively with striatal [18F]fluorodopa uptake, but these associations did not reach statistical significance in the linear regression model. In contrast to irritability, two other aggressivity-related scales (indirect aggression and verbal aggression) had no clear relationships with [18F]fluorodopa uptake.
There was no significant association between the score on the detachment scale and [18F]fluorodopa uptake in any of the striatal regions. Two other extraversion scales that are supposedly related to novelty-seeking behavior (monotony avoidance and impulsiveness) did not have any consistent relationship with [18F]fluorodopa uptake. Scales measuring psychopathy versus conformity (socialization and social desirability) had consistently positive correlations, and hostility-related scales (suspicion and guilt) had consistently negative correlation coefficients in all striatal regions, but none of these associations reached statistical significance in the linear regression model. No statistically significant associations were observed between [18F]fluorodopa uptake in the putamen and any of the personality scales with the linear regression model.
Because of multiple comparisons, we also performed conventional Bonferroni analysis of the results from linear regression analysis. After adjustment for 60 comparisons, none of the associations remained significant. However, when we used averages of [18F]fluorodopa uptake in the left and right hemispheres and adjusted for 30 comparisons, the associations for the 33 healthy subjects between uptake in the caudate and scores for somatic anxiety (r=− 0.59, p=0.03, Bonferroni-corrected p value), muscular tension (r= − 0.60, p=0.03), and irritability (r=− 0.59, p=0.03) still remained significant (Figure 1). Caution should therefore be used in interpretation of results from the left and right hemispheres separately. None of the associations for the putamen was significant in this analysis.
We also compared the results from linear regression analysis to those from Pearson’s correlation analysis using personality scores that were transformed to T scores according to normative data. The 33 subjects’ scores on both the somatic anxiety and muscular tension scales also correlated negatively with [18F]fluorodopa uptake in the right caudate in this analysis (r=−0.46, p=0.007, and r=−0.47, p=0.007, respectively). In addition, two other anxiety-related scales, psychic anxiety and psychasthenia, also correlated negatively with [18F]fluorodopa uptake in the right caudate (r=−0.40, p=0.02, and r=−0.58, p=0.0004, respectively), and psychasthenia also correlated with uptake in the left putamen (r=−0.49, p=0.004). A negative correlation between irritability and [18F]fluorodopa uptake was also observed in the right caudate, although with lower statistical significance (r=−0.37, p=0.04). With this method, the positive correlation between socialization and [18F]fluorodopa uptake became highly significant in the right caudate and left putamen (r=0.54, p=0.002, and r=0.52, p=0.002, respectively).
Discussion
The most robust finding in the present study was the consistent association between low [18F]fluorodopa uptake in the caudate and high scores on anxiety-related scales of the Karolinska Scales of Personality. This correlation was statistically significant for the somatic anxiety and muscular tension scales. Stronger correlations for somatic than for psychic anxiety scales—psychic anxiety, psychasthenia, and inhibition of aggression (also referred to as lack of assertiveness)—may indicate that the dopaminergic system is involved mainly in autonomic manifestations of anxiety. To our knowledge, no significant associations between anxiety-related traits and uptake of other dopaminergic PET tracers have been observed (Table 1). The positive correlation between D2 receptor binding potential and the neuroticism factor of the Revised NEO Personality Inventory in the study by Breier and co-workers (12) was attributed to the depression facet, not to anxiety, and may be of different etiology. However, our observation could be related to psychiatric symptoms of patients with Parkinson’s disease. Up to 40% of patients with Parkinson’s disease suffer from anxiety disorders (26), and anxiety symptoms are shown to improve with levodopa therapy (27). Rapid and dose-dependent reduction of anxiety symptoms even after acute levodopa infusion was observed in patients with Parkinson’s disease in a placebo-controlled, double-blind study (28). Moreover, the prevalence of anxiety disorders in subjects who later develop Parkinson’s disease is already increased 5–20 years before the onset of motor symptoms (29). Since the emergence of motor symptoms generally requires at least a 50% loss of nigrostriatal dopaminergic neurons (30), the premorbid increase in the prevalence of anxiety disorders in Parkinson’s disease patients could be due to a neurologically subclinical but already active pathological process of dopaminergic neurodegeneration. However, the neurobiological substrate for anxiety may differ between normal healthy subjects and patients with Parkinson’s disease (22), and at this point this link is still hypothetical.
Genetic studies have not provided many data on the specific genes involved in the development of anxiety-related personality traits. It is interesting that an association between allelic variation in activating protein type 2β (AP-2β), a transcription factor, and several anxiety-related scales of the Karolinska Scales of Personality has been reported (31). AP-2β is expressed in the midbrain during brain development and to some extent throughout life (32), and several monoaminergic genes, including the dopa decarboxylase gene, have AP-2 binding sites in their regulatory regions (33). Specifically, women homozygous for a longer allele (five instead of four [CAAA]n repeats in the second intron) had lower scores on the muscular tension, somatic anxiety, and psychasthenia scales than those with one or two copies of the shorter allele (31). However, the functional significance of this intronic polymorphism is not known.
Another strong association observed in our study was between low [18F]fluorodopa uptake in the caudate and a high irritability score, although the correlations of uptake with other aggressivity-related scales did not reveal any consistent pattern. Aggression is a nonspecific and both psychologically and neurobiologically heterogenous emotion. However, Farde and colleagues (9) reported a trend-level (r=−0.51, p<0.01) negative correlation between irritability score and D2 dopamine receptor density in the putamen. Together, these two independent observations suggest that low dopaminergic neurotransmission may indeed be associated with some personality characteristics measured with this scale. It contains items such as “Sometimes people bother me just by being around” and “I can’t help being a little rude to people I don’t like.” Furthermore, high scores on the “angry hostility” facet of the neuroticism scale of the Eysenck Personality Questionnaire have been associated with one of the alleles of the tyrosine hydroxylase gene (eight [TCAT]n repeats) (34). Since tyrosine hydroxylase activity may directly affect [18F]fluorodopa uptake (see following discussion), these findings may well be related.
The social desirability scale showed a trend-level positive correlation with [18F]fluorodopa uptake in the right putamen when we used the linear regression model. We reported previously (13) a significant positive correlation between social desirability and dopamine transporter binding in the right putamen. Since these two studies were independent, this finding warrants further research. Subjects scoring high on the social desirability scale are supposed to be good listeners, always helpful, etc. (“No matter whom I’m talking to, I’m always polite and courteous,” “I have never been bothered when someone has asked me for a favor, not even at times when it has been inconvenient”). The other scale from the cluster measuring psychopathy versus conformity, socialization, also showed a significant and positive correlation with [18F]fluorodopa uptake in the right caudate and left putamen in the Pearson’s correlation analysis but only a tendency and only in left putamen when the linear regression model was applied. Although there is a lot of support for the involvement of the brain dopamine system in social behaviors (as discussed at the beginning of the article), it must be noted that the relationship between [18F]fluorodopa uptake and scales measuring social interactions was less marked (and more dependent on the type of statistical test used) than the relationship between [18F]fluorodopa and anxiety-related traits. The fact that high striatal [18F]fluorodopa uptake is also associated with schizophrenic psychosis (23, 35) suggests either a nonlinear (U-shaped) relationship between social functioning and subcortical dopaminergic activity, or a different causal relationship between dopaminergic activity and behavior in normal and psychotic subjects.
We did not find any significant associations between the detachment scale and [18F]fluorodopa uptake. In the right putamen, where the negative correlation between dopamine transporter binding and detachment scores was strongest in our previous study (13), the present study still gave results pointing in the same direction. However, while [18F]fluorodopa uptake and dopamine transporter binding can both be conceived as presynaptic markers of dopaminergic neurons, they reflect different aspects of neuronal function. Two other extraversion-related scales, impulsiveness and monotony avoidance, did not show any association either. These scales are the closest to Cloninger’s novelty-seeking trait, which has been extensively studied for its putative association with the human D4 dopamine receptor gene (10, 11). There have also been single reports (36–38) suggesting associations between this trait and genes for the D2 and D3 dopamine receptors and the dopamine transporter. Although our results do not support an association between novelty-seeking behavior and presynaptic dopamine synthesis capacity in healthy subjects, it must be noted that the Karolinska Scales of Personality may be a less sensitive instrument than the Tridimensional Personality Questionnaire or the Temperament and Character Inventory for measuring this trait (39).
It is also important to note that the significance and nature of interindividual differences in striatal [18F]fluorodopa uptake in healthy subjects are not fully understood. [18F]Fluorodopa uptake is supposed to reflect neuronal dopamine synthesis rate, or “capacity,” but the rate-limiting step in dopamine synthesis is actually tyrosine hydroxylase, not dopa decarboxylase. However, there exists indirect evidence suggesting that [18F]fluorodopa uptake really indicates the rate of dopamine synthesis. Stimulation of tyrosine hydroxylase activity in experimental animals by cofactor 6R-l-erythro-5,6,7,8,-tetrahydrobiopterin and l-tyrosine infusion increases striatal l-[β-11C]dopa (an analog of [18F]fluorodopa) uptake (40), as well as its conversion to [11C]dopamine and the release of [11C]dopamine to the extracellular space as measured with in vivo microdialysis (41). These findings suggest that the increased activity of dopaminergic neurons, leading to increased dopaminergic neurotransmission, can also be observed as increased [18F]fluorodopa uptake.
In conclusion, our data suggest that anxiety-related personality traits are associated with low presynaptic dopamine synthesis capacity in the caudate as assessed by PET and [18F]fluorodopa in healthy human subjects. In addition, the data are consistent with the previously reported negative correlation between irritability scale scores and dopaminergic markers. Possible positive correlations between traits related to social behaviors and dopaminergic activity warrant further research. Whether related correlations exist in extrastriatal brain regions, such as limbic cortical structures with functional connections to the caudate (42), will also be an interesting question for future imaging studies to answer. Moreover, as the net efficacy of dopaminergic neurotransmission depends on both pre- and postsynaptic activity, assessing several variables (e.g., D1 and D2 dopamine receptors, dopamine transporters, fluorodopa uptake) in the same subjects would likely provide a more unified view of the role of the dopaminergic system in human personality and behavior.
 |
 |
Received April 22, 2002; revision received Nov. 13, 2002; accepted Nov. 25, 2002. From the Department of Pharmacology and Clinical Pharmacology, University of Turku, Turku, Finland; the Accelerator Laboratory and Neuropsychiatric Imaging, Turku PET Centre; the Department of Psychiatry, Turku University Central Hospital; and the Digital Media Institute, Institute of Signal Processing, Tampere University of Technology, Tampere, Finland. Address reprint requests to Dr. Hietala, Neuropsychiatric Imaging, Turku PET Centre, Turku University Central Hospital, Kiinamyllynkatu 4-8, 20520 Turku, Finland; [email protected] (e-mail).
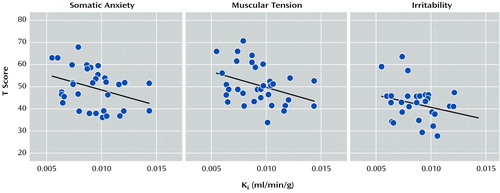
Figure 1. Correlations Between [18F]Fluorodopa Uptake Ki Values in the Caudate and T Scores for Somatic Anxiety, Muscular Tension, and Irritability on the Karolinska Scales of Personality for 33 Healthy Subjectsa
aPart of the variance seen in these figures is due to the age- and sex-related variation in [ 18F]fluorodopa uptake.
1. Cloninger CR: A systematic method for clinical description and classification of personality variants: a proposal. Arch Gen Psychiatry 1987; 44:573-588Crossref, Medline, Google Scholar
2. Cloninger CR, Svrakic DM, Przybeck TR: A psychobiological model of temperament and character. Arch Gen Psychiatry 1993; 50:975-990Crossref, Medline, Google Scholar
3. López-Ibor JJ Jr: The concept and boundaries of personality disorders. Am J Psychiatry 1997; 154(June festschrift suppl):21-25Google Scholar
4. Schalling D, Åsberg M, Edman G, Oreland L: Markers for vulnerability to psychopathology: temperament traits associated with platelet MAO activity. Acta Psychiatr Scand 1987; 76:172-182Crossref, Medline, Google Scholar
5. Costa PT Jr, McCrae RR: The NEO Personality Inventory Manual. Odessa, Fla, Psychological Assessment Resources, 1985Google Scholar
6. Eysenck HJ, Eysenck MW: Personality and Individual Differences: A Natural Science Approach. New York, Plenum, 1985Google Scholar
7. Stallings MC, Hewitt J-K, Cloninger CR, Heath AC, Eaves LJ: Genetic and environmental structure of the Tridimensional Personality Questionnaire: three to four temperament dimensions. J Pers Soc Psychol 1996; 70:127-140Crossref, Medline, Google Scholar
8. Limson R, Goldman D, Roy A, Lamparski D, Ravitz B, Adinoff B, Linnoila M: Personality and cerebrospinal fluid monoamine metabolites in alcoholics and controls. Arch Gen Psychiatry 1991; 48:437-441Crossref, Medline, Google Scholar
9. Farde L, Gustavsson JP, Jönsson E: D2 dopamine receptors and personality traits (letter). Nature 1997; 385:590Crossref, Medline, Google Scholar
10. Ebstein RP, Benjamin J, Belmaker RH: Personality and polymorphisms of genes involved in aminergic neurotransmission. Eur J Pharmacol 2000; 410:205-214Crossref, Medline, Google Scholar
11. Paterson AD, Sunohara GA, Kennedy JL: Dopamine D4 receptor gene: novelty or nonsense? Neuropsychopharmacology 1999; 21:3-16Crossref, Medline, Google Scholar
12. Breier A, Kestler L, Adler C, Elman I, Wiesenfeld N, Malhotra A, Pickar D: Dopamine D2 receptor density and personal detachment in healthy subjects. Am J Psychiatry 1998; 155:1440-1442Link, Google Scholar
13. Laakso A, Vilkman H, Kajander J, Bergman J, Haaparanta M, Solin O, Hietala J: Prediction of detached personality in healthy subjects by low dopamine transporter binding. Am J Psychiatry 2000; 157:290-292Link, Google Scholar
14. Kestler LP, Malhotra AK, Finch C, Adler C, Breier A: The relation between dopamine D2 receptor density and personality: preliminary evidence from the NEO Personality Inventory—Revised. Neuropsychiatry Neuropsychol Behav Neurol 2000; 13:48-52Medline, Google Scholar
15. Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, Suzuki K: Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. Neuroimage 2001; 13:891-895Crossref, Medline, Google Scholar
16. Tiihonen J, Kuikka J, Bergström K, Lepola U, Koponen H, Leinonen E: Dopamine reuptake site densities in patients with social phobia. Am J Psychiatry 1997; 154:239-242Link, Google Scholar
17. Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin S-H, Laruelle M: Low dopamine D2 receptor binding potential in social phobia. Am J Psychiatry 2000; 157:457-459Link, Google Scholar
18. Gustavsson JP, Weinryb RM, Göransson S, Pedersen NL, Åsberg M: Stability and predictive ability of personality traits across 9 years. Pers Individ Diff 1997; 22:783-791Crossref, Google Scholar
19. Gustavsson JP, Pedersen NL, Åsberg M, Schalling D: Origins of individual differences in anxiety proneness: a twin/adoption study of the anxiety-related scales from the Karolinska Scales of Personality (KSP). Acta Psychiatr Scand 1996; 93:460-469Crossref, Medline, Google Scholar
20. Gustavsson JP, Pedersen NL, Åsberg M, Schalling D: Exploration into the sources of individual differences in aggression-, hostility- and anger-related (AHA) personality traits. Pers Individ Diff 1996; 21:1067-1071Crossref, Google Scholar
21. Menza MA, Mark MH, Burn DJ, Brooks DJ: Personality correlates of [18F]dopa striatal uptake: results of positron-emission tomography in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1995; 7:176-179Crossref, Medline, Google Scholar
22. Kaasinen V, Nurmi E, Bergman J, Eskola O, Solin O, Sonninen P, Rinne JO: Personality traits and brain dopaminergic function in Parkinson’s disease. Proc Natl Acad Sci USA 2001; 98:13272-13277Crossref, Medline, Google Scholar
23. Hietala J, Syvälahti E, Vilkman H, Vuorio K, Räkköläinen V, Bergman J, Haaparanta M, Solin O, Kuoppamäki M, Eronen E, Ruotsalainen U, Salokangas RK: Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res 1999; 35:41-50Crossref, Medline, Google Scholar
24. Patlak CS, Blasberg RG, Fenstermacher JD: Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983; 3:1-7Crossref, Medline, Google Scholar
25. Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvälahti E, Salokangas RKR, Hietala J: Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry 2002; 52:759-763Crossref, Medline, Google Scholar
26. Richard IH, Schiffer RB, Kurlan R: Anxiety and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1996; 8:383-392Crossref, Medline, Google Scholar
27. Fetoni V, Soliver P, Monza D, Testa D, Girotti F: Affective symptoms in multiple system atrophy and Parkinson’s disease: response to levodopa therapy. J Neurol Neurosurg Psychiatry 1999; 66:541-544Crossref, Medline, Google Scholar
28. Maricle RA, Nutt JG, Valentine RJ, Carter JH: Dose-response relationship of levodopa with mood and anxiety in fluctuating Parkinson’s disease: a double-blind, placebo-controlled study. Neurology 1995; 45:1757-1760Crossref, Medline, Google Scholar
29. Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA: Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case-control study. Mov Disord 2000; 15:669-677Crossref, Medline, Google Scholar
30. Guttman M, Burkholder J, Kish SJ, Hussey D, Wilson A, DaSilva J, Houle S: [11C]RTI-32 PET studies of the dopamine transporter in early dopa-naive Parkinson’s disease: implications for the symptomatic threshold. Neurology 1997; 48:1578-1583Crossref, Medline, Google Scholar
31. Damberg M, Garpenstrand H, Alfredsson J, Ekblom J, Forslund K, Rylander G, Oreland L: A polymorphic region in the human transcription factor AP-2β gene is associated with specific personality traits. Mol Psychiatry 2000; 5:220-224Crossref, Medline, Google Scholar
32. Moser M, Imhof A, Pscherer A, Bauer R, Amselgruber W, Sinowatz F, Hofstadter F, Schule R, Buettner R: Cloning and characterization of a second AP-2 transcription factor: AP-2β. Development 1995; 121:2779-2788Medline, Google Scholar
33. Hahn SL, Hahn M, Kang UJ, Joh TH: Structure of the rat aromatic L-amino acid decarboxylase gene: evidence for an alternative promoter usage. J Neurochem 1993; 60:1058-1064Crossref, Medline, Google Scholar
34. Persson M-L, Wasserman D, Jönsson EG, Bergman H, Terenius L, Gyllander A, Neiman J, Geijer T: Search for the influence of the tyrosine hydroxylase (TCAT)n repeat polymorphism on personality traits. Psychiatry Res 2000; 95:1-8Crossref, Medline, Google Scholar
35. Hietala J, Syvälahti E, Vuorio K, Räkköläinen V, Bergman J, Haaparanta M, Solin O, Kuoppamäki M, Kirvelä O, Ruotsalainen U, Salokangas RKR: Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenia patients. Lancet 1995; 346:1130-1131Crossref, Medline, Google Scholar
36. Noble EP, Ozkaragoz TZ, Ritchie TL, Zhang X, Belin TR, Sparkes RS: D2 and D4 dopamine receptor polymorphisms and personality. Am J Med Genet 1998; 81:257-267Crossref, Medline, Google Scholar
37. Thome J, Weijers HG, Wiesbeck GA, Sian J, Nara K, Boning J, Riederer P: Dopamine D3 receptor gene polymorphism and alcohol dependence: relation to personality rating. Psychiatr Genet 1999; 9:17-21Crossref, Medline, Google Scholar
38. Sabol SZ, Nelson ML, Fisher C, Gunzerath L, Brody CL, Hu S, Sirota LA, Marcus SE, Greenberg BD, Lucas FR 4th, Benjamin J, Murphy DL, Hamer DH: A genetic association for cigarette smoking behavior. Health Psychol 1999; 18:7-13Crossref, Medline, Google Scholar
39. Jönsson EG, Nöthen MM, Gustavsson JP, Neidt H, Brené S, Tylec A, Propping P, Sedvall GC: Lack of evidence for allelic association between personality traits and the dopamine D4 receptor gene polymorphisms. Am J Psychiatry 1997; 154:697-699Link, Google Scholar
40. Tsukada H, Lindner K-J, Hartvig P, Tani Y, Valtysson J, Bjurling P, Kihlberg T, Westerberg G, Watanabe Y, Langström B: Effect of 6R-l-erythro-5,6,7,8-tetrahydrobiopterin and infusion of l-tyrosine on the in vivo l-[β-11C]DOPA disposition in the monkey brain. Brain Res 1996; 713:92-98Crossref, Medline, Google Scholar
41. Tsukada H, Lindner K-J, Hartvig P, Tani Y, Bjurling P, Kihlberg T, Westerberg G, Watanabe Y, Langström B: Effect of 6R-l-erythro-5,6,7,8-tetrahydrobiopterin on in vivo l-[β-11C]DOPA turnover in the rat striatum with infusion of l-tyrosine. J Neural Transm Gen Sect 1994; 95:1-15Crossref, Medline, Google Scholar
42. Parent A: Extrinsic connections of the basal ganglia. Trends Neurosci 1990; 13:254-258Crossref, Medline, Google Scholar



