A Collaborative Study of the Emergence and Clinical Features of the Major Depressive Syndrome of Alzheimer’s Disease
Abstract
OBJECTIVE: This report provides a description of the prevalence and clinical features of the major depressive syndrome of Alzheimer’s disease using data derived from structured diagnostic assessments of 243 patients with probable Alzheimer’s disease and 151 nondemented elderly comparison subjects. METHOD: Subjects were characterized by a consortium of four Alzheimer’s disease research centers and the Geriatric Psychiatry Branch of the National Institute of Mental Health. All sites administered the Clinical Assessment of Depression in Dementia, a structured, anchored diagnostic interview that was developed to reliably diagnose and characterize major depressive episodes in this population. RESULTS: Despite the use of a common, reliable methodology for the assessment and diagnosis of major depressive episodes, the prevalence of major depression in Alzheimer’s disease ranged widely from 22.5% to 54.4% across the recruitment sites. The prevalence of major depressive episodes among Alzheimer’s disease patients in the aggregate sample exceeded that for elderly comparison subjects and reached nearly 50% among the most severely demented patients. Alzheimer’s disease patients with a current major depressive episode had earlier mean ages at onset, a higher mean Hamilton Depression Rating Scale score, and were more likely to be experiencing psychotic symptoms than those who had not developed a major depressive episode. Although the major depressive episodes of Alzheimer’s disease patients and nondemented elderly comparison subjects included similar numbers of depressive symptoms, patients with Alzheimer’s disease were more likely to report a diminished ability to concentrate or indecisiveness and less likely to experience sleep disturbances and feelings of worthlessness or excessive guilt during their major depressive episodes. None of the clinical features of major depression differed significantly in frequency among depressed Alzheimer’s disease patients with mild, moderate, or severe dementia. Concurrent psychotic symptoms progressively increased with dementia severity. CONCLUSIONS: The high rate of major depressive episodes that occur after the onset of cognitive impairment among patients with Alzheimer’s disease (the majority of whom had no premorbid history of major depression), common emergence in the early stages of dementia when symptoms of cognitive impairment are least likely to contribute to the syndromal diagnosis of major depression, and differences in the clinical presentations of the major depressive episodes of Alzheimer’s disease patients and nondemented elderly comparison subjects, all support the validity of the major depressive syndrome of Alzheimer’s disease. Our findings suggest that the major depressive syndrome of Alzheimer’s disease may be among the most common mood disorders of older adults.
Alzheimer’s disease is the most common cause of dementia among the elderly (1–3). The defining features of Alzheimer’s disease include progressive, global cognitive impairment that emerges in individuals whose brains develop densities of senile plaques that exceed those expected for age (4, 5). However, patients with this disorder manifest remarkable interindividual variability in other clinical characteristics, including age at symptomatic onset; rate and pattern of progression; emergence of disturbances of mood, thought, perception, and behavior; development of extrapyramidal symptoms; and the presence of a family history of Alzheimer’s disease-like dementia. Postmortem and genetic studies have also revealed considerable interindividual variation in comorbid neuropathological findings, brain neurochemical abnormalities, and contributions from particular genetic factors. This level of heterogeneity suggests that Alzheimer’s disease, as currently defined, may more closely resemble a syndrome with multiple contributing etiologies than a disease with a unitary cause (6).
Clinically significant depression is a common and important complication of Alzheimer’s disease that increases the suffering of patients and their families, produces excess disability, promotes institutionalization, and hastens death (7, 8). Estimates of the prevalence of depression among patients with Alzheimer’s disease have ranged from 0% to 86%, with most values clustering in the range of 30% to 50% (8–10). This wide range of estimates of comorbidity may arise in part from differences in the populations surveyed, in the assessment methodologies employed, and in the diagnostic criteria applied. Depression is also common in other types of degenerative dementia, including those that arise from Parkinson’s disease, Huntington’s disease, Pick’s disease, and in dementias of vascular origin(9).
Degeneration of the major brainstem aminergic nuclei that occurs in Alzheimer’s disease is likely to contribute to disturbances in perception, mood, thought, and behavior (“noncognitive” disturbances) (6, 9, 10). Projections from the dorsal and median raphe nuclei provide extensive serotonergic innervation of the forebrain. The noradrenergic cells of the locus ceruleus project axons widely to both the neocortex and the hippocampus. Alzheimer’s disease is associated with the loss of neuronal cells from both of these nuclei, and a substantial fraction of those that remain develop neurofibrillary tangles (9, 11–14). The neurochemical correlates of this process include decrements in the levels of these amine neurotransmitters and their metabolites, their respective biosynthetic enzymes, and in the presynaptic reuptake of both neurotransmitters in their projection areas (15–19).
Published neuropathological and neurochemical studies suggest that patients with Alzheimer’s disease who develop the syndrome of major depression in the context of dementia comprise a clinically and pathologically distinct subgroup of patients who have degenerative changes in the brainstem aminergic nuclei (especially the locus ceruleus) that are disproportionate to those that occur in the cerebral cortex, and have relative preservation of the cholinergic neurons in the basal forebrain that innervate the hippocampus and neocortex (9, 19–22). This last observation is interesting in the context of Alzheimer’s disease, since the progression of the central cholinergic deficit that occurs in this disorder may interact with the pathophysiology of depression to limit the development of major depressive episodes in later stages of this disorder (10, 19, 23). Several lines of evidence from published autopsy studies suggest that these neuropathological and neurochemical correlates of major depression in Alzheimer’s disease have relative specificity for this mood disorder and differ from those associated with psychosis, exposure to psychotropic medications, and the neurodegeneration in Alzheimer’s disease more generally (9, 10, 19–24). Preliminary evidence suggests that neuronal loss in the aminergic nuclei of Alzheimer’s disease patients involves apoptotic events (10) and that the variation in neuronal loss in these brain areas may be influenced by susceptibility loci that also affect the risk of developing Alzheimer’s disease (10, 25–28).
This report describes the initial results of a collaborative, clinicopathologic study of the major depressive syndrome of Alzheimer’s disease funded by the National Institute of Mental Health (NIMH) and the National Institute on Aging (NIA) (grant MH/AG-47346). Research subjects with probable Alzheimer’s disease and nondemented comparison subjects were recruited and characterized by a consortium of four NIA-funded Alzheimer’s disease research centers and the Geriatric Psychiatry Branch of NIMH. Clinical assessments of major depression were performed with the Clinical Assessment of Depression in Dementia (CADD), a structured, anchored diagnostic interview that was developed by this collaborative group to reliably diagnose and characterize the number and course of major depressive episodes in this population. The CADD incorporates information gathered from the patient, the caregiver or other best informant, and available medical records. This report provides a description of the prevalence and clinical features of the major depressive syndrome of Alzheimer’s disease using data derived from the assessments of 243 cognitively impaired patients with probable Alzheimer’s disease and 151 nondemented elderly comparison subjects who were recruited and characterized by this consortium.
Method
Subject Recruitment and Characterization
Subjects for this research project were recruited and characterized by a consortium of four Alzheimer’s disease research centers located at the University of California at Los Angeles (UCLA) (AG-16570), the Mayo Clinic (AG-16574), Indiana University (AG-10133), and Mt. Sinai School of Medicine (AG-05138) as well as the Geriatric Psychiatry Branch of NIMH. Subjects were recruited from cohorts of demented patients, who fulfilled the clinical criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association for probable Alzheimer’s disease (29), and neurologically healthy elderly comparison subjects, who were being followed longitudinally at each recruitment site on an annual basis, or more frequently if clinically indicated. Demented and comparison subjects characterized at the UCLA, Indiana, and NIMH sites were recruited through a variety of outreach activities including advertisements in the local media and educational presentations made in the community, as well as through referrals made by family members, caregivers, physicians, and local advocacy groups. Demented and comparison subjects characterized at the Mt. Sinai site were primarily recruited through systematic screening of residents at a large nursing home. Demented and comparison subjects characterized at the Mayo Clinic site were recruited through the systematic screening of patient contacts made through the Department of Medicine, Division of Community Medicine, one of the largest providers of primary care in the region. Approximately 50% of the comparison subjects recruited at the Indiana site were spouses of Alzheimer’s disease patients, while the rates of spousal comparison subjects recruited at the remaining sites were similar to the population rate. Written informed consent was obtained from subjects with Alzheimer’s disease who were able to provide it, or their next of kin, and all comparison subjects prior to participation at each recruitment site. This research project was approved by the Institutional Review Boards of all participating institutions.
Complete diagnostic evaluations were performed for patients and comparison subjects by American Board of Psychiatry and Neurology-certified neurologists and psychiatrists at each site prior to entry. These evaluations included complete histories and physical examinations (including mental status and neurological examinations), as well as a standardized battery of laboratory, neuroimaging, and neuropsychological assessments as recommended by the Quality Standards Subcommittee of the American Academy of Neurology (30). These assessments were typically augmented by medical records provided by the subjects’ primary care physicians and any other diagnostic assessments performed prior to recruitment. At entry and at subsequent longitudinal assessments, evaluations of the cognitive performance of patients and comparison subjects included the Mini-Mental State Examination (MMSE) (31). Functional capacity and impairment were also rated at these time points with the Clinical Dementia Rating (32), using information provided by caregivers.
Potential comparison subjects with any history or current evidence of cognitive decline or other neurological symptoms were excluded. Demented patients who had a history of a neurological disease other than Alzheimer’s disease prior to the onset of cognitive decline, or evidence of any neurological disease other than Alzheimer’s disease at the time of the initial evaluation, were similarly excluded. Clinical diagnoses and best estimates of the age of symptomatic onset of dementia were established at regular consensus conferences at which all available clinical information was reviewed. The age at symptomatic onset of dementia was defined by the emergence of the first evidence of cognitive or functional impairment.
Assessment of Depression
Clinical assessments of major depression were performed using the CADD, a structured diagnostic interview that was designed to diagnose and characterize the number, onset, and course of major depressive episodes in this population (available from the corresponding author). The CADD incorporates a structured, anchored version of the 17-item Hamilton Depression Rating Scale that has been validated for use in this population (33); the subsection of the Neuropsychiatric Inventory (34) that assesses the presence/absence, frequency, and severity of a list of hallucinations and delusions that are commonly experienced by patients with dementia; and the portion of the Structured Clinical Interview for DSM-III-R (SCID) (35) that scores signs and symptoms required for the diagnosis of major depression into a single coherent clinical interview that can be completed in approximately 30 minutes. The DSM-III-R symptom criteria for the diagnosis of a major depressive episode are identical to those included in DSM-III and DSM-IV.
Signs and symptoms of depression were scored using the inclusive method for the diagnosis of major depression without the need to interpret whether a clinical feature was attributable to the dementia, a task that is often difficult to achieve with confidence. This approach to the syndromal diagnosis of major depression in Alzheimer’s disease has been validated by both neuropathological and neurochemical studies of postmortem brain tissue (9, 10, 19–24) and has been employed in numerous studies of the prevalence and symptomatic presentation, natural history/course, functional status/excess disability, and treatment response (7, 8, 10).
Responses to each item were elicited from each subject, a best informant/caregiver, and included a final judgment by the trained clinician who conducted the interview and who had also reviewed available medical records. Raters were encouraged to explore and resolve any apparent inconsistencies that were encountered. This level of clinical judgment required that the raters be clinicians who were experienced in the assessment of older subjects with cognitive and other mental disorders. Supervision by faculty psychiatrists was provided at each site. When discrepancies occurred, they were often (but not always) the result of underreporting by the demented patient who was unaware or did not remember the symptoms reported by the informant. Such discrepancies were typically resolved by reports from informants who described observable events and consequences, with timelines, treatment interventions, and treatment outcomes. Medical records were often helpful in substantiating these accounts or suggesting additional symptoms or behavioral disturbances for review with the patient and informant. Patients with Alzheimer’s disease occasionally reported symptoms, such as disturbances of perception, that were not observable and of which the informant and medical caregivers were unaware. If identified discrepancies could not be resolved with a reasonable degree of confidence, the respective variables were coded as unknown. The CADD also recorded sociodemographic information, current medications, age at onset of cognitive decline, both MMSE score and Clinical Dementia Rating at the time of assessment, and a narrative summary describing the evaluator’s diagnostic assessment.
Training sessions at each site were organized by the project coordinator at the University of Pittsburgh and the principal rater at each participating site. Training consisted of didactic instruction on the format and content of the CADD, the anchor points used for rating symptoms, completion of the score sheets and a brief narrative summary, followed by an opportunity for the clarification of questions. Raters also viewed a videotaped CADD interview with an Alzheimer’s disease patient and best informant, performed by an experienced geriatric psychiatrist. After another question and answer period, raters performed five audiotaped CADD interviews with patients and informants, reviewed available medical records, and completed the CADD scoring sheets and narrative. These materials, stripped of personal identifiers, were reviewed and critiqued by an expert rater at the University of Pittsburgh, who provided feedback to each rater. This process continued until acceptable reliability against the standard was achieved.
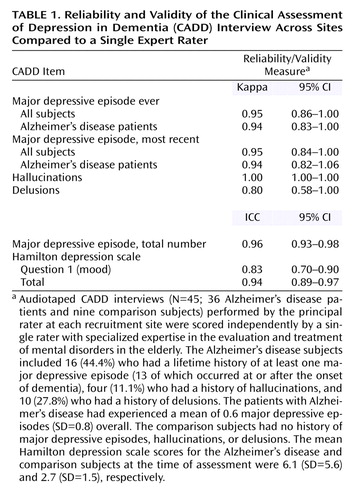
Following training sessions, audiotaped CADD interviews (N=45) performed by the principal rater at each recruitment site were scored independently by a single expert rater at the University of Pittsburgh with specialized expertise in the evaluation and treatment of mental disorders in the elderly. All research materials were stripped of personal identifiers before they were sent to the University of Pittsburgh. As shown in Table 1, estimates of the reliability of the diagnostic assessments and related clinical information elicited using the CADD were excellent. Kappa statistics reflected the performance of raters across sites.
Statistical Analysis
The interrater reliability of diagnostic assessments and clinical variables determined from structured CADD interviews was evaluated using the kappa statistic and the intraclass correlation coefficient, respectively. Mean values were compared using the t statistic, or an analysis of variance (ANOVA) followed by Tukey post hoc honestly significant difference tests. Univariate relationships between clinical variables and the prevalence of major depression in Alzheimer’s disease across recruitment sites were explored using the Pearson correlation coefficient or Spearman rho statistic. A general linear model procedure was used to provide a regression analysis and ANOVA to evaluate the independent effects of recruitment site and major depression on the age at onset of cognitive impairment among patients with Alzheimer’s disease. Proportions and rates were compared using the chi-square statistic or Fisher’s exact test, as appropriate. All tests were two-tailed. Statistical analyses were performed using the software packages SPSS version 10.0 (SPSS, Inc., Chicago) or MedCalc version 5.0 (MedCalc Software, Mariakerke, Belgium).
Results
A clinical description and comparison of the 243 Alzheimer’s disease patients and 151 nondemented elderly comparison subjects is presented in Table 2. The Alzheimer’s disease patients included an excess of women, experienced the onset of cognitive impairment at a mean age of 69.0, and manifested a moderate level of cognitive and functional impairment on average as reflected by mean MMSE score and Clinical Dementia Rating of 18.0 and 1.6, respectively. Nearly half of the patients with Alzheimer’s disease had experienced at least one major depressive episode during their lifetimes, and approximately one-third developed major depressive episodes at or after the onset of cognitive impairment. Their mean Hamilton depression scale score of 7.2 was modestly elevated relative to comparison subjects, primarily reflecting the elevated Hamilton depression scale scores of the 44 Alzheimer’s disease patients (18.1%) who were experiencing a current major depressive episode at the time of assessment. Twenty-one (25.0%) of the 84 Alzheimer’s disease patients who developed a major depressive episode at or after the onset of cognitive impairment had a premorbid history of major depression, compared to 24 (15.1%) of Alzheimer’s disease patients who did not develop a major depressive episode in the context of cognitive impairment (χ2=3.40, df=1, p=0.07), a trend that is consistent with a previous study of psychiatric inpatients with Alzheimer’s disease (36). The corresponding rates of premorbid “late-onset” major depression (onset of first major depressive episode at or after age 50), 14.3% and 8.9%, did not discriminate between the Alzheimer’s disease groups who developed or lacked a major depressive episode at or after the onset of dementia (χ2=1.64, df=1, p=0.20). Approximately one-fifth of the Alzheimer’s disease patients had experienced delusions, typically of the simple and paranoid type, and one-tenth had experienced hallucinations.
The nondemented elderly comparison subjects had a similar sex ratio but were younger than the demented patients. Their lack of cognitive impairment was reflected by a mean MMSE score and Clinical Dementia Rating that approached the optimal scores of 30 and 0, respectively. None of the comparison subjects had any history of psychotic symptoms, although approximately one-quarter of them had suffered from major depression.
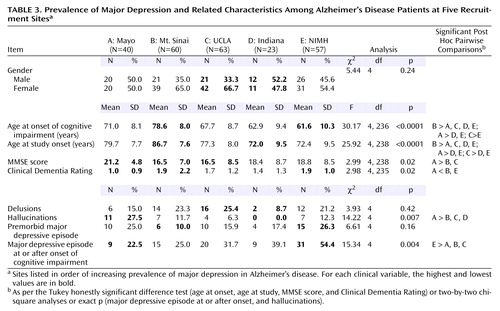
Recruitment sites are listed in Table 3 in order of increasing prevalence of major depression among Alzheimer’s disease patients, which ranged significantly from 22.5% (Mayo) to 54.4% (NIMH). For each clinical variable, the highest and lowest values are highlighted. Between-site differences in the groups of Alzheimer’s disease patients that were studied included mean ages at symptomatic onset and recruitment, mean MMSE score and Clinical Dementia Rating, and prevalence of hallucinations, although the ranges of values across recruitment sites were often small to moderate in size. On average, the patients recruited at Mayo were the least cognitively impaired, the most likely to manifest hallucinations, and the least likely to suffer from major depression; those from Mt. Sinai experienced the latest onset of cognitive decline, were the oldest at the time of study, and exhibited the greatest cognitive impairment; those from UCLA tied with patients from Mt. Sinai for the greatest cognitive impairment as indicated by the MMSE; those from Indiana were the youngest at the time of study and were least likely to exhibit hallucinations; and patients from NIMH experienced the earliest onset of cognitive impairment, tied for the greatest impairment as reflected by the Clinical Dementia Rating, and were the most likely to suffer from major depression.
Of the variables listed in Table 3, only age at onset of cognitive decline exhibited a significant negative association with the prevalence of major depression across the five recruitment sites (rs=–0.90, p=0.04). A general linear model was employed to evaluate the relationship of age at onset of cognitive impairment to the emergence of major depression and recruitment site. In this model, only recruitment site emerged as an independent predictor of the variance in age at onset (F=23.46, df=4, 231, p<0.001), while the association of major depression with age at onset approached statistical significance (F=3.33, df=1, 231, p=0.07). No interaction between recruitment site and major depression was detected (F=0.42, df=4, 231, p=0.79). Interestingly, the prevalence of major depression among the Alzheimer’s disease patients recruited at sites that were based in Departments of Neurology (UCLA, Mayo, and Indiana) did not differ significantly from that of Alzheimer’s disease patients who were recruited at sites that were psychiatry based (Mt. Sinai and NIMH): 30.2% (N=38 of 126) and 41.9% (N=49 of 117), respectively. However, a trend favoring a higher prevalence of major depression among the demented patients recruited at the psychiatry-based recruitment sites was observed (χ2=3.63, df=1, p=0.06).
The proportions of Alzheimer’s disease patients who experienced the onset of a major depressive episode at or after the onset of cognitive impairment were calculated for subgroups of Alzheimer’s disease patients with increasing levels of dementia severity, as reflected by MMSE scores (Figure 1). Consistent with previous reports (23, 37), the largest increment in the proportion of patients who experienced a major depressive episode at or after the onset of cognitive impairment (25.9%) occurred among the patients with the mildest cognitive impairment. The prevalence of major depression gradually increased to nearly 50% in the Alzheimer’s disease subjects with the most severe cognitive impairment, as reflected by MMSE scores in the lowest quartile.
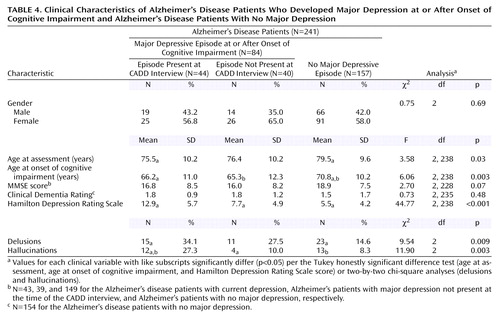
A comparison of Alzheimer’s disease patients who developed major depression and those who did not is presented in Table 4. Forty-four (52.4%) of the 84 patients who developed a major depressive episode in the context of cognitive impairment were experiencing a current episode at the time of the CADD interview. These individuals had earlier mean ages at onset of cognitive impairment and study onset, a higher mean Hamilton depression scale score, and were more likely to be experiencing delusions and hallucinations than Alzheimer’s disease patients who had not developed a major depressive episode in the context of their dementias. Patients with Alzheimer’s disease who had developed a major depressive episode, but were not suffering from a current episode, generally manifested clinical features that were intermediate in severity or prevalence between those with a current episode and those who had not developed a major depressive episode in the context of dementia.
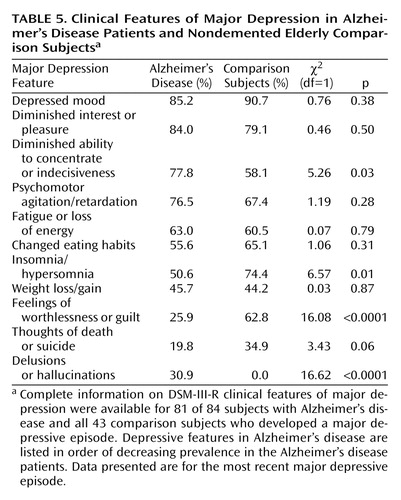
A comparison of the clinical features of the most recent major depressive episodes experienced by the Alzheimer’s disease patients and those of the nondemented elderly comparison subjects is presented in Table 5. The depressive features are listed in order of decreasing frequency among the patients, followed by the frequency of psychotic symptoms (delusions or hallucinations). The mean number of depressive features for the major depressive episodes experienced by the Alzheimer’s disease patients (mean=6.8, SD=1.5) was similar to the corresponding mean for the elderly comparison subjects (mean=6.4, SD=1.4) (t=1.68, df=1.22, p=0.10). In contrast, the distribution of depressive features differed significantly between the groups. During their most recent major depressive episode, patients with Alzheimer’s disease were significantly more likely to report diminished ability to concentrate or indecisiveness and significantly less likely to report sleep disturbances (insomnia/hypersomnia) and feelings of worthlessness or excessive guilt. The last difference is especially noteworthy, since feelings of worthlessness or excessive guilt are a common feature of major depression among cognitively intact elders, but affected only a minority of Alzheimer’s disease patients with major depression in our sample. Recurrent thoughts of death or suicidal ideation also tended to be less common among Alzheimer’s disease patients with major depression, a finding that is consistent with a previous study of elderly psychiatric inpatients (38). In addition to these depressive features, none of the elderly comparison subjects developed psychotic symptoms during their major depressive episodes, compared to nearly a third of Alzheimer’s disease patients who experienced delusions or hallucinations during their major depressive episodes.
A comparison of the clinical features of major depression in Alzheimer’s disease patients with mild, moderate, and severe cognitive impairment is presented in Table 6. The mean numbers of depressive features for these three groups (mean=6.6 [SD=1.8], mean=6.3 [SD=1.3], and mean=6.7 [SD=1.9], respectively) were similar (F=0.27, df=2, 76, p=0.76). Furthermore, no significant changes in the distribution of depressive features were observed across the groups. A trend toward increased frequency of psychomotor agitation/retardation and fatigue/loss of energy was noted for the most cognitively impaired patients. In contrast, the proportion of depressed patients with psychotic symptoms (delusions or hallucinations) increased significantly with increasing cognitive impairment. Similar results were obtained when the sample was dichotomized by the median MMSE score.
Discussion
This report describes the initial results of a collaborative clinicopathologic study of the major depressive syndrome of Alzheimer’s disease. These efforts began with the design and pilot testing of a structured, anchored, diagnostic interview that could be used to reliably diagnose and characterize the number and course of major depressive episodes in samples of Alzheimer’s disease patients and comparison subjects across participating sites. The CADD fulfilled this need and represents the first structured diagnostic interview with demonstrated reliability to diagnose and characterize the number and course of major depressive episodes in this population, using information gathered from the patient, the caregiver or other best informant, and available medical records. This assessment tool should facilitate a wide range of research efforts that address the etiology, clinical biology, epidemiology, natural history, treatment, health services utilization, and the economic consequences of this common and important clinical complication of Alzheimer’s disease. This goal was among the research priorities identified by a recent Workshop on the Depression of Alzheimer’s Disease, sponsored by the Adult and Geriatric Treatment and Preventive Interventions Research Branch of NIMH (8).
Despite the use of a common, reliable methodology for the assessment and diagnosis of major depressive episodes, the prevalence of major depression in Alzheimer’s disease ranged widely from 22.5% (Mayo) to 54.4% (NIMH) across the recruitment sites. This site-related variance in prevalence rates was not explained by differences in the ages of the Alzheimer’s disease patients at the time of study, dementia severity as measured by MMSE score and Clinical Dementia Rating, the prevalence of delusions or hallucinations, or the presence of premorbid histories of major depression. Although the prevalence of major depression across sites was associated with an earlier age at onset of cognitive decline, this univariate association only approached statistical significance in a model that controlled for the effect of recruitment site.
It seemed plausible that Alzheimer’s disease patients with psychiatric complications including major depression would be more likely to be evaluated at recruitment sites based in university Departments of Psychiatry or the NIMH Geriatric Psychiatry Branch than those based in Departments of Neurology. While the results for NIMH and Mayo Clinic were consistent with this hypothesis, the recruitment efforts from all sites revealed only a modest increase in the prevalence of major depression in Alzheimer’s disease at the psychiatry-based recruitment sites (41.9%) compared to the neurology-based sites (30.2%), a difference that only approached statistical significance. Therefore, the source of the substantial variation in the prevalence of major depression in Alzheimer’s disease across recruitment sites remains largely unexplained. Additional potential sources of referral bias and the possibility of bona fide geographic differences in the rates of major depression and other clinical or preclinical features of Alzheimer’s disease warrant further consideration. The latter may provide clues to potential factors that may contribute to Alzheimer’s disease and its clinical manifestations. Regardless of the basis for the variation in the prevalence of major depression in Alzheimer’s disease across sites, the prevalence of this clinical complication was high, affecting almost half of patients in our sample who had reached the most severe stages of dementia.
Patients with Alzheimer’s disease who were experiencing a current major depressive episode at the time of the CADD interview had earlier mean ages at onset of illness and study, a higher mean Hamilton depression scale score, and were more likely to be experiencing delusions and hallucinations than Alzheimer’s disease patients who had not developed a major depressive episode in the context of their dementias. Not surprisingly, the average severity of the major depressive episodes experienced by these outpatients, reflected by a mean Hamilton depression scale of 12.9, was modest by comparison to inpatients with Alzheimer’s disease described in a previous study (23) whose major depressive episodes were evaluated using the same structured, anchored Hamilton depression scale (N=37, mean Hamilton depression scale score=20.7), and patients who are typically recruited to participate in treatment trials for depression (8). However, 54.5% (N=24 of 44) of the Alzheimer’s disease patients in our study who were suffering from a current major depressive episode were receiving treatment with an antidepressant medication for this condition. Treatment is likely to have contributed to a partial reduction in their Hamilton depression scale scores, which reflect the severity of their symptoms during the 2-week period prior to the CADD interview. Alternatively, the major depressive episodes that occur in the context of Alzheimer’s disease may tend to be less severe than those that occur in cognitively intact individuals, perhaps as the result of different underlying etiologies. In contrast to the Hamilton depression scale scores, treatment would not have affected the number or frequency of depressive symptoms that occurred during the major depressive episodes as determined by the CADD (SCID subsection), which elicits signs and symptoms of depression that were present during the entire episode (e.g., Table 5 and Table 6). It is tempting to speculate that the higher frequency of psychotic symptoms among Alzheimer’s disease patients with current major depressive episodes may have been related to elevations in hippocampal dopamine levels, as reported in a previous neurochemical study of postmortem brain tissue (19).
The lifetime prevalence rates of major depression among the Alzheimer’s disease patients and nondemented elderly comparison subjects in our study were 44.9% and 28.5%, respectively. Both of these estimates were objectively high and greatly in excess of the 1.4% figure reported by the Epidemiologic Catchment Area study of community-dwelling, nondemented elders (39). The high prevalence of premorbid major depressive episodes (18.5%) in the Alzheimer’s disease patients is consistent with published evidence suggesting that premorbid major depressive episodes increase the risk of developing Alzheimer’s disease, while the 34.6% prevalence of major depressive episodes that occurred in the context of dementia may be related to neurodegenerative events that contribute to the etiology of major depression among Alzheimer’s disease patients. Elevated rates of depression are common among caregivers of Alzheimer’s disease patients and may have contributed to the high prevalence of major depression among the comparison subjects in our study.
Although the most recent major depressive episodes of Alzheimer’s disease patients and nondemented elderly comparison subjects included similar numbers of depressive symptoms, the distributions of depressive symptoms differed significantly between these groups. Patients with Alzheimer’s disease were significantly more likely to report a diminished ability to concentrate or indecisiveness, a difference that could conceivably have been attributable to their cognitive impairment at the earliest stages of dementia. Patients with Alzheimer’s disease were significantly less likely to experience sleep disturbances and feelings of worthlessness or excessive guilt during their major depressive episodes than nondemented elderly comparison subjects. These last two differences do not appear to be attributable to cognitive impairment for different reasons. The presence/absence of sleep disturbances are observable phenomena, and the reports of the Alzheimer’s disease patients were generally corroborated by caregivers/best informants during the CADD interviews. Although they cannot be measured objectively, the frequency of feelings of worthlessness or excessive guilt remained essentially constant (23.1%–30.0%) (Table 6) during the major depressive episodes of Alzheimer’s disease patients regardless of the severity of dementia from very mild to severe. In fact, none of the clinical features of major depression, except concurrent psychosis, changed significantly in frequency among the Alzheimer’s disease patients with mild, moderate, or severe dementia. This observation makes cognitive impairment an unlikely mechanism to explain the reduced frequency of this clinical feature of major depression among patients with Alzheimer’s disease. Both cognitive impairment and a reduced frequency of feelings or worthlessness or excessive guilt may have contributed to the trend toward a reduced frequency of morbid or suicidal ideation.
The high rate of major depressive episodes that occur at or after the onset of cognitive impairment among patients with Alzheimer’s disease (the majority of whom had no premorbid history of major depression), the common emergence in the early stages of dementia when symptoms of cognitive impairment are least likely to contribute to the syndromal diagnosis of major depression, and differences in the clinical presentations of the major depressive episodes of Alzheimer’s disease patients and nondemented elderly comparison subjects all support the validity of the major depressive syndrome of Alzheimer’s disease. Our findings suggest that this mood syndrome may affect approximately one-third of the 4 million Americans who currently suffer from Alzheimer’s disease, a rate that may exceed 50% by the late stages of dementia. Current population estimates suggest that Alzheimer’s disease affects 8% to 15% of individuals over the age of 65 (40), and the prevalence is growing as our national population ages. In contrast, the incidence and prevalence of major depression among cognitively normal older Americans declines with age (39–41), and prevalence estimates of major depression in cognitively normal older adults have been 5% or less (39, 42, 43). These estimates suggest that the major depressive syndrome of Alzheimer’s disease may be among the most common mood disorders of late life.
 |
 |
 |
 |
 |
 |
Received June 7, 2002; revisions received Aug. 17 and Sept. 19, 2002; accepted Oct. 4, 2002. From the Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh; the Department of Biological Sciences, Mellon College of Science, Carnegie-Mellon University, Pittsburgh; the Department of Neurology, UCLA School of Medicine, Los Angeles; the Geriatric Psychiatry Branch, National Institute of Mental Health, Bethesda, Md.; the Department of Psychiatry, Mt. Sinai School of Medicine, New York; the Department of Neurology, Indiana University School of Medicine, Indianapolis; and the Department of Neurology, Mayo Clinic, Rochester, Minn. Address correspondence to George S. Zubenko, M.D., Ph.D., Western Psychiatric Institute and Clinic, Room E1230, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). This research was supported by research project grant MH/AG-47346 from the National Institute of Mental Health, and Alzheimer’s Disease Research Center grants AG-16570, AG-16574, AG-10133, and AG-05138 from the National Institute on Aging. G.S.Z. was the recipient of Independent Scientist Award MH-00540 from the National Institute of Mental Health.
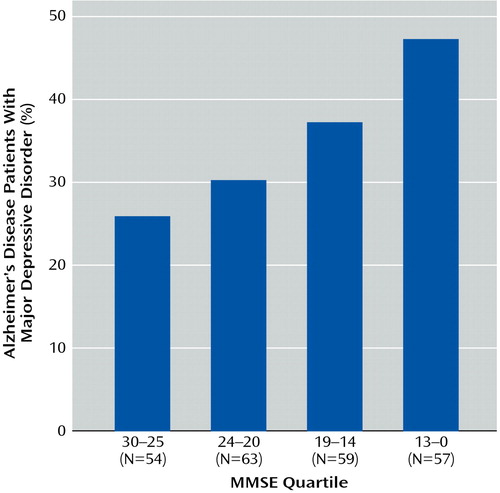
Figure 1. Major Depressive Disorder Onset at or After the Onset of Cognitive Impairment in Alzheimer’s Disease Patients by Level of Dementia Severitya
aRates for decreasing MMSE quartiles were 25.9%, 30.2%, 37.3%, and 47.4%, respectively.
1. Jorm AF, Korten AE, Henderson AS: The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 1987; 76:465-479Crossref, Medline, Google Scholar
2. Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO: Prevalence of Alzheimer’s disease in a community population of older persons: higher than previously reported. JAMA 1989; 262:2551-2556Crossref, Medline, Google Scholar
3. Bachman DL, Wolf PA, Linn R, Konefel JE, Cobb J, Belanger A, D’Agostino RB, White LR: Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology 1992; 42:115-119Crossref, Medline, Google Scholar
4. Khachaturian ZS: Diagnosis of Alzheimer’s disease. Arch Neurol 1985; 42:1097-1105Crossref, Medline, Google Scholar
5. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991; 41:479-486Crossref, Medline, Google Scholar
6. Zubenko GS: Molecular neurobiology of Alzheimer’s disease (syndrome?). Harv Rev Psychiatry 1997; 5:177-213Crossref, Medline, Google Scholar
7. Lebowitz B (section editor): Older adults and mental health, in Mental Health: A Report of the Surgeon General. Rockville, Md, US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, National Institutes of Health, National Institute of Mental Health, 1999, pp 335-401 Google Scholar
8. Olin JT, Katz IR, Meyers BS, Schneider LS, Lebowitz B: Provisional diagnostic criteria for depression of Alzheimer disease: rationale and background. Am J Geriatr Psychiatry 2002; 10:129-141Crossref, Medline, Google Scholar
9. Zubenko GS, Moossy J: Major depression in primary dementia: clinical and neuropathologic correlates. Arch Neurol 1988; 45:1182-1186Crossref, Medline, Google Scholar
10. Zubenko GS: Neurobiology of major depression in Alzheimer’s disease. Int Psychogeriatr 2000; 12:217-230Crossref, Google Scholar
11. Tomlinson BE, Irving D, Blessed G: Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J Neurol Sci 1981; 49:419-428Crossref, Medline, Google Scholar
12. Iversen LL, Rossor MN, Reynolds GP, Hills R, Roth M, Mountjoy CQ, Foote SL, Morrison JH, Bloom FE: Loss of pigmented dopamine-β-hydroxylase positive cells from locus coeruleus in senile dementia of Alzheimer’s type. Neurosci Lett 1983; 39:95-100Crossref, Medline, Google Scholar
13. Curcio CA, Kemper T: Nucleus raphe dorsalis in dementia of the Alzheimer type: neurofibrillary changes and neuronal packing density. J Neuropathol Exp Neurol 1984; 43:359-368Crossref, Medline, Google Scholar
14. Yamamoto T, Hirano A: Nucleus raphe dorsalis in Alzheimer’s disease: neurofibrillary tangles and loss of large neurons. Ann Neurol 1985; 17:573-577Crossref, Medline, Google Scholar
15. Cross AJ, Crow TJ, Perry EK, Perry RH, Blessed G, Tomlinson BE: Reduced dopamine-beta-hydroxylase activity in Alzheimer’s disease. Br Med J 1981; 1:93-94Crossref, Google Scholar
16. Winblad B, Adolfsson R, Carlsson A, Gottfries C-G: Biogenic amines in brains of patients with Alzheimer’s disease, in Alzheimer’s Disease: A Report of Progress in Research (Aging). Edited by Corkin S, Davis KL, Growdon JH, Usdin E, Wurtman RJ. New York, Raven Press, 1982, pp 25-33Google Scholar
17. Bowen DM, Allen SJ, Benton JS, Goodhardt MF, Haan EA, Palmer AM, Sims NR, Smith CC, Spillane JA, Esiri MM, Neary D, Snowdon JS, Wilcock GK, Davison AN: Biochemical assessment of serotonergic and cholinergic dysfunction and cerebral atrophy in Alzheimer’s disease. J Neurochem 1983; 41:266-272Crossref, Medline, Google Scholar
18. Rossor M, Iversen LL: Non-cholinergic neurotransmitter abnormalities in Alzheimer’s disease. Br Med Bull 1986; 42:70-74Crossref, Medline, Google Scholar
19. Zubenko GS, Moossy J, Kopp U: Neurochemical correlates of major depression in primary dementia. Arch Neurol 1990; 47:209-214Crossref, Medline, Google Scholar
20. Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL: The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol 1988; 24:233-242Crossref, Medline, Google Scholar
21. Chan-Palay V: Depression and senile dementia of the Alzheimer type: catecholamine changes in the locus coeruleus—basis for therapy. Dementia 1990; 1:253-261Google Scholar
22. Forstl H, Burns A, Luthert P, Cairns N, Lantos P, Levy R: Clinical and neuropathological correlates of depression in Alzheimer’s disease. Psychol Med 1992; 22:877-884Crossref, Medline, Google Scholar
23. Zubenko GS, Rosen J, Sweet RA, Mulsant BH, Rifai AH: Impact of psychiatric hospitalization on behavioral complications of Alzheimer’s disease. Am J Psychiatry 1992; 149:1484-1491Link, Google Scholar
24. Zubenko GS, Moossy J, Martinez AJ, Rao G, Claassen D, Rosen J, Kopp U: Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol 1991; 48:619-624Crossref, Medline, Google Scholar
25. Zubenko GS, Hughes HB, Stiffler JS: Clinical and neurobiological correlates of D10S1423 genotype in Alzheimer’s disease. Biol Psychiatry 1999; 46:740-749Crossref, Medline, Google Scholar
26. Zubenko GS, Hughes HB, Stiffler JS: Clinical and neurobiological correlates of DXS1047 genotype in Alzheimer’s disease. Biol Psychiatry 1999; 46:173-181Crossref, Medline, Google Scholar
27. Zubenko GS, Hughes HB, Stiffler JS: Neurobiological correlates of a putative risk allele for Alzheimer’s disease on chromosome 12q. Neurology 1999; 52:725-732Crossref, Medline, Google Scholar
28. Zubenko GS: Do susceptibility loci contribute to the expression of more than one mental disorder? a view from the genetics of Alzheimer’s disease (millennium article). Mol Psychiatry 2000; 5:131-136Crossref, Medline, Google Scholar
29. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939-944Crossref, Medline, Google Scholar
30. Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC: Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143-1153Crossref, Medline, Google Scholar
31. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
32. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566-572Crossref, Medline, Google Scholar
33. Mulsant BH, Sweet R, Rifai AH, Pasternak R, McEachran A, Zubenko GS: The use of the Hamilton Rating Scale for Depression in elderly patients with cognitive impairment and physical illness. Am J Geriatr Psychiatry 1994; 2:220-229Crossref, Medline, Google Scholar
34. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308-2314Crossref, Medline, Google Scholar
35. Spitzer RL, Williams JBW, Gibbon M, First MB: Instruction Manual for the Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1988Google Scholar
36. Zubenko GS, Rifai AH, Mulsant BH, Sweet RA, Pasternak RE: Premorbid history of major depression and the depressive syndrome of Alzheimer’s disease. Am J Geriatr Psychiatry 1996; 4:85-90Medline, Google Scholar
37. Fischer P, Simanyi M, Danielczyk W: Depression in dementia of the Alzheimer type and in multi-infarct dementia. Am J Psychiatry 1990; 147:1484-1487Link, Google Scholar
38. Rifai AH, Mulsant BH, Sweet RA, Pasternak RE, Rosen J, Zubenko GS: A study of elderly suicide attempters admitted to an inpatient psychiatric unit. Am J Geriatr Psychiatry 1993; 1:126-135Crossref, Medline, Google Scholar
39. Weissman MM, Bruce ML, Leaf PJ, Florio LP, Holzer C: Affective disorders, in Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Edited by Robins LN, Regier DA. New York, Free Press, 1991, pp 53-80Google Scholar
40. Ritchie K, Kildea D: Is senile dementia “age-related” or “aging-related”? evidence from meta-analysis of dementia prevalence in the oldest old. Lancet 1995; 346:931-934Crossref, Medline, Google Scholar
41. Romanoski AJ, Folstein MF, Nastadt G, Chahal R, Merchant A, Brown CH, Gruenberg EM, McHugh PR: The epidemiology of psychiatrist-ascertained depression and DSM-III depressive disorders: results from the Eastern Baltimore Mental Health Survey Clinical Reappraisal. Psychol Med 1992; 22:629-655Crossref, Medline, Google Scholar
42. Eaton WW, Anthony JC, Gallo J, Cai G, Tien A, Romanoski A, Lyketsos C, Chen LS: Natural history of Diagnostic Interview Schedule/DSM-IV major depression: the Baltimore Epidemiologic Catchment Area follow-up. Arch Gen Psychiatry 1997; 54:993-999Crossref, Medline, Google Scholar
43. Gurland BJ, Cross PS, Katz S: Epidemiologic perspectives on opportunities for treatment of depression. Am J Geriatr Psychiatry 1996; 4(suppl 1):S7-S13Google Scholar



