Inferior Frontal White Matter Anisotropy and Negative Symptoms of Schizophrenia: A Diffusion Tensor Imaging Study
Abstract
OBJECTIVE: The purpose of this study was test the hypothesis that abnormalities of inferior frontal white matter are related to the negative symptoms of schizophrenia. METHOD: Fractional anisotropy of white matter tracts in the prefrontal area of 10 schizophrenic patients was determined by diffusion tensor imaging. Patients were also assessed for severity of negative symptoms by using the Schedule for the Assessment of Negative Symptoms (SANS). RESULTS: Inferior frontal white matter fractional anisotropy was significantly inversely correlated with the SANS global ratings of negative symptoms. CONCLUSIONS: These data, while preliminary, suggest that impaired white matter integrity in the inferior frontal region may be associated with the severity of negative symptoms in schizophrenia.
Brain imaging studies (1–6) have demonstrated volumetric white matter deficits (primarily in the frontal lobe and in the corpus callosum) among patients with schizophrenia. In addition, several studies have used diffusion tensor imaging to specifically characterize potential CNS white matter abnormalities in schizophrenia. (Although not absolutely correlated with morphological structure, the displacement distribution of water as measured by diffusion tensor imaging can provide information regarding microstructure, organization, and integrity of tissue [7].) Four of six studies using diffusion tensor imaging (8–13) have indicated differences in diffusion measures suggestive of abnormalities in white matter structure in schizophrenia.
Data from structural magnetic resonance imaging (MRI) studies in schizophrenia have further indicated that white matter abnormalities may be specifically associated with negative symptoms (2–4); these data include our prior finding (3) that patients with high levels of negative symptoms have the lowest white matter volume in inferior regions. Diffusion tensor imaging is a useful modality for probing white matter structures, and we know of no studies that have used this method to examine the potential relationship of frontal white matter microstructure and negative symptoms. Therefore, we conducted diffusion tensor imaging studies of 10 patients with schizophrenia who had varying degrees of negative symptoms. These studies were performed in order to test the hypothesis that negative symptoms are associated with abnormalities in inferior frontal white matter.
Method
After complete description of the study to the subjects, written informed consent was obtained from 10 male schizophrenic in- and outpatients. The patients were all under 50 years of age and met the DSM-IV criteria for schizophrenia. Patients with depression meeting diagnostic criteria, drug abuse/dependence within the last year, or history of neurological disease were excluded. The subjects were selected on the basis of a priori clinical familiarity in order to obtain a range of negative symptom severity. All subjects were receiving antipsychotic medication. The clinical rating measures included the Brief Psychiatric Rating Scale (BPRS) (14) and the Schedule for Assessment of Negative Symptoms (SANS) (15). Ratings were obtained when the patients were considered to be at maximal clinical stability (“baseline”), up to 40 days after the MRI scan. For the statistical analyses, negative symptom ratings were reduced to the SANS subscale global scores, a single total of the SANS global subscores, and the score on the withdrawal-retardation factor from the BPRS.
To minimize artifacts induced by eddy currents, the diffusion tensor imaging data were acquired by using a double-echo pulsed-gradient echo planar imaging method without cardiac gating, with TR=6 sec, TE=100 msec, field of view=24 cm, 128×128 matrix reconstructed to 256×256, b=1000 sec/mm2, four averages, six noncollinear directions aligned to the anterior-posterior commissure (AC-PC) plane, and acquisition time=2 minutes, 36 seconds. Fractional anisotropy and mean diffusivity were computed as described in a previous report (16). All raw and processed images were examined for artifacts by a reviewer who was blind to subject identity (K.O.L. or S.J.C.).
Standardized circular regions of interest were positioned, by an investigator blind to subject identity (S.J.C.), on the T2-weighted image (b=0) of the diffusion tensor imaging data set; thus, coregistration of images across different MRI acquisition methods was not needed. A single region of interest was placed bilaterally in the frontal white matter of five consecutive 5-mm-thick slices. These slices included three that were above the index slice, the index slice itself (defined as the most inferior slice containing the genu of the corpus callosum—approximately 10 mm above the AC-PC plane), and one slice below. For the three most superior frontal slices, large regions of interest (area=84.4 mm2) were centered in white matter with the posterior boundary of each region of interest on the imaginary line formed across the anterior most tips of the two frontal horns of the lateral ventricles. For the two most inferior frontal slices, medium-sized regions of interest (area=43.5 mm2) were centered in white matter with the posterior boundary of the region of interest on the imaginary line linking the most lateral extent of the sylvian fissures across the hemispheres.
Spearman’s rank order correlation coefficients were calculated to assess the relationship of negative symptom variables with white matter fractional anisotropy and mean diffusivity in the five frontal regions. To reduce the number of comparisons, we averaged fractional anisotropy and mean diffusivity across hemispheres to yield one fractional anisotropy and mean diffusivity value per slice. For these analyses, alpha was set at 0.01, two-tailed. The reported results represent the only hypothesis regarding anatomic regions and symptom scores that was tested.
Results
The mean age of the 10 patients was 41 years (SD=9); their mean duration of illness was 17 years (SD=9), and their mean sum of SANS global subscores was 12.8 (range=9–18).
Across subjects, frontal white matter fractional anisotropy at 5 mm below the AC-PC plane was inversely correlated with the severity of affective blunting (r=–0.72, p=0.02) and anhedonia (r=–0.69, p=0.03), according to the SANS subscales, and with a higher overall sum of SANS global subscores (r=–0.84, p=0.002) (Figure 1). Similarly, greater withdrawal-retardation, indicated by the BPRS, was inversely correlated with white matter fractional anisotropy in the same inferior frontal region (r=–0.76, p=0.02).
Discussion
The main finding of this study is that fractional anisotropy in the inferior frontal white matter is lower among patients with more severe negative symptoms than among other patients with schizophrenia. This finding is consistent with those of our previous structural MRI study (3), in which significant inverse correlations were found between the severity of negative symptoms and prefrontal white matter volumes. These findings suggest that orbitofrontal white matter microstructure may play an important role in the pathophysiology of negative symptoms in schizophrenia by disruption of cortical-cortical or cortical-subcortical connectivity within neuronal networks. Further elucidation of such abnormalities may shed light on the “anatomy” of negative symptoms. Given the limitations of this preliminary study, these results also need to be replicated with a larger number of subjects.
Received March 18, 2002; revision received Sept. 3, 2002; accepted Sept. 6, 2002. From the Mental Health Service, VA New York Harbor Healthcare System; and the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y. Address reprint requests to Dr. Wolkin, Mental Health Service (11M), VA New York Harbor Healthcare System, 423 East 23rd St., New York, NY 10010; [email protected] (e-mail). Supported by the Department of Veterans Affairs and by NIMH grant MH-60662.
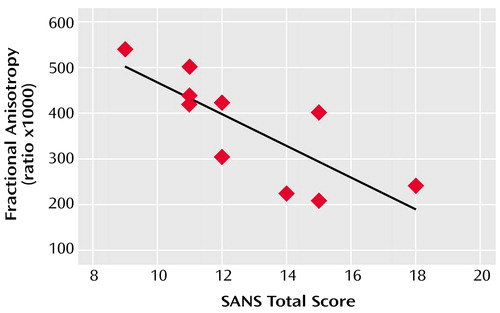
Figure 1. Relationship Between Fractional Anisotropy of Inferior Frontal White Matter and the Sum of Global Subscores on the Scale for Assessment of Negative Symptoms (SANS) for 10 Men With Schizophrenia
1.. Woodruff PW, McManus IC, David AS: Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry 1995; 58:457-461Crossref, Medline, Google Scholar
2.. Tibbo P, Nopoulos P, Arndt S, Andreasen NC: Corpus callosum shape and size in male patients with schizophrenia. Biol Psychiatry 1998; 44:405-412Crossref, Medline, Google Scholar
3.. Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Feiner D, Rotrosen J, Wolkin A: Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry 2000; 57:471-480Crossref, Medline, Google Scholar
4.. Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, Levitt JJ, O’Donnell BF, Kikinis R, Jolesz FA, McCarley RW: Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res 2001; 108:65-78Crossref, Medline, Google Scholar
5.. Wolkin A, Rusinek H, Vaid G, Arena L, Lafargue T, Sanfilipo M, Loneragan C, Lautin A, Rotrosen J: Structural magnetic resonance image averaging in schizophrenia. Am J Psychiatry 1998; 155:1064-1073Link, Google Scholar
6.. Sigmundsson T, Suckling J, Maier M, Williams SCR, Bullmore ET, Greenwood KE, Fukuda R, Ron MA, Toone BK: Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 2001; 158:234-243Link, Google Scholar
7.. Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H: Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001; 13:534-546Crossref, Medline, Google Scholar
8.. Agartz I, Andersson JL, Skare S: Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. Neuroreport 2001; 12:2251-2254Crossref, Medline, Google Scholar
9.. Steel RM, Bastin ME, McConnell S, Marshall I, Cunningham-Owens DG, Lawrie SM, Johnstone EC, Best JJ: Diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (1H MRS) in schizophrenic subjects and normal controls. Psychiatry Res 2001; 106:161-170Crossref, Medline, Google Scholar
10.. Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA: Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 2000; 68:242-244Crossref, Medline, Google Scholar
11.. Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A: Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry 1999; 56:367-374Crossref, Medline, Google Scholar
12.. Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, Downhill J, Haznedar M, Fallon JH, Atlas SW: MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport 1998; 9:425-430Crossref, Medline, Google Scholar
13.. Foong J, Symms MR, Barker GJ, Maier M, Miller DH, Ron MA: Investigating regional white matter in schizophrenia using diffusion tensor imaging. Neuroreport 2002; 13:333-336Crossref, Medline, Google Scholar
14.. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
15.. Andreasen NC: Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry 1982; 39:784-788Crossref, Medline, Google Scholar
16.. Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP: Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry 2002; 51:890-895Crossref, Medline, Google Scholar



