Inhibitory Effect of Hippocampal 5-HT1A Receptors on Human Explicit Memory
Abstract
OBJECTIVE: Recent studies have indicated that the serotonergic (5-HT) system plays important roles in memory function. However, the specific relationship between 5-HT1A receptors and memory function is not clear in the human brain. To clarify this relationship, the authors determined the availability of 5-HT1A receptors in the human brain and the relationship between regional receptor binding and memory function. METHOD: Using positron emission tomography (PET) with [11C]WAY-100635, the authors examined 5-HT1A receptors and assessed their relationship with memory function. The 5-HT1A agonist tandospirone was then administered to investigate the effect of 5-HT1A receptor stimulation on cognitive function and neuroendocrinological response. RESULTS: There was a significant negative correlation between explicit memory function and 5-HT1A receptor binding localized in the bilateral hippocampus where the postsynaptic 5-HT1A receptors are enriched. Furthermore, the administration of tandospirone dose-dependently impaired explicit verbal memory, while other cognitive functions showed no significant changes. The change in memory function paralleled those of body temperature and secretion of growth hormone, which were reported to be induced by the stimulation of postsynaptic 5-HT1A receptors. CONCLUSIONS: Postsynaptic 5-HT1A receptors localized in the hippocampal formation have a negative influence on explicit memory function, which raises the possibility that the antagonistic effect of postsynaptic 5-HT1A receptors in the hippocampus leads to improvement of human memory function. Drugs that work as antagonists on postsynaptic 5-HT1A receptors may be favorable for improved control of memory impairment.
Memory is a highly complex process that involves several brain structures as well as the role of several neurotransmitters. Considerable efforts have been devoted to the development of a successful neurotransmitter-based pharmacotherapy for the memory dysfunction found in neurological and psychiatric diseases. Cholinergic and glutamatergic systems have been linked to cognitive processes such as attention, learning, and mnemonic function (1, 2). However, accumulating evidence from recent studies indicates that other neurotransmitter systems, such as the serotonergic (5-HT) system, play a role in behaviors that involve high cognitive demand (3).
Among the 5-HT receptor subtypes shown to play a role in various learning and memory models, the 5-HT1A receptors are of particular interest. This receptor is characterized by its high concentration in the limbic system (4), such as the hippocampus, which is known to play an important role in learning and memory (5, 6). It interacts with other neurotransmitter systems, such as in the glutamatergic and cholinergic systems (7, 8). In animal studies, specific agonists and antagonists of the 5-HT1A receptor showed a consistent role for this receptor in learning and memory function (4).
Several lines of evidence have suggested that the 5-HT1A receptor may be involved in the pathophysiology of schizophrenia and the mechanism of action of atypical antipsychotic drugs (9–11). Atypical antipsychotic drugs with partial agonistic property on 5-HT1A receptors were reported to improve verbal memory function in patients with schizophrenia (12–14). The use of a small dose of the 5-HT1A agonist tandospirone in patients with schizophrenia has been reported to improve verbal memory (15, 16). However, the exact mechanisms underlying these effects are not yet well defined, and the relationship in vivo in humans between the 5-HT1A receptors and cognitive function across levels from underlying brain systems to neurons and cellular events within these systems has not been clarified.
To gain an understanding of this relationship, we performed positron emission tomography (PET) scans using [11C]WAY-100635 (17, 18) to examine the 5-HT1A receptor, assessing the relationship between regional receptor binding and memory function. To interpret the pharmacological implications, we administered the 5-HT1A agonist tandospirone (19–21) to subjects and investigated the effect of the stimulation of 5-HT1A receptors on cognitive function together with the neuroendocrinological response of growth hormone (GH) and body temperature, which reflect postsynaptic 5-HT1A receptor activity (22–24).
Method
Subjects
The subjects were 16 healthy male volunteers aged 21–48 years (mean=28.7, SD=6.7) who did not meet criteria for any neuropsychiatric disorders and did not have relatives with neuropsychiatric disorders according to unstructured psychiatric screening interviews. T1-weighted magnetic resonance images (MRI) revealed no brain abnormalities. Memory performance of each subject was evaluated with the Wechsler Memory Scale—Revised (WMS-R) (25, 26). This study was approved by the Ethics and Radiation Safety Committee of the National Institute of Radiological Sciences, Chiba, Japan. Written informed consent was obtained from all subjects.
PET Study Procedure
After a transmission scan with a 68Ge-68Ga source, a 176.5–237.9-MBq bolus injection of [carbonyl-11C]WAY-100635 (denoted here as [11C]WAY-100635) was administered with a specific radioactivity of 42.6–400.0 GBq/μmol at injection. Radioactivity was measured for 90 minutes with a CTI-Siemens ECAT EXACT HR+ scanner (CTI-Siemens, Knoxville, Tenn.) in three-dimensional mode, which provides 63 planes and a 15.5-cm field of view. All emission scans were reconstructed with a Hanning filter cutoff frequency of 0.4 (full width at half maximum=7.5 mm). T1-weighted MRI was acquired by Phillips Intera, 1.5 tesla. T1-weighted images of the brain were obtained from all subjects. The scan parameters were 1 mm-thick three-dimensional T1 images with a transverse plane (TR=21 msec, TE=9.2 msec, flip angle=30°, matrix=256×256, field of view=256×256 mm).
Quantification of 5-HT1A Receptors
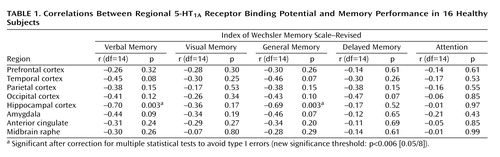
Radioactivity in nine brain regions (cerebellum, anterior cingulate cortex, prefrontal cortex, temporal cortex, parietal cortex, occipital cortex, amygdala, hippocampal cortex, and midbrain raphe) was obtained with a template-based method for defining regions of interest described in our previous study (27) with the exception of the midbrain raphe. The location of the region of interest for the midbrain raphe nuclei region was defined by using the method described in the study by Drevets et al. (28). The circular region of interest (5 mm radius) was centered over the midbrain raphe nuclei evident on PET summation images. Midbrain sections were identified in the coregistered MRI. The average values of right and left regions of interest were used to increase the signal-to-noise ratio for the calculations. [11C]WAY-100635 binding was quantified with a reference tissue compartmental model with the cerebellum used as reference tissue (29). This model allows the estimation of binding potential, which is defined as follows: binding potential (k3/k4)=f2 Bmax/(Kd [1 + Σi Fi/Kdi]), where k3 and k4 describe the exchange of tracer between the free compartment and a specifically bound ligand compartment, f2 is the “free fraction” of unbound radioligand, Bmax the density of receptor, Kd the dissociation constant for the radioligand, and Fi and Kdi are the free concentration and the dissociation constant of the competing endogenous ligand, respectively. Pearson correlation coefficients were obtained for the binding potential values of each region and values of WMS-R composites. A p value <0.006 (0.05/8) was considered significant for the avoidance of type I errors in the multiplicity of statistical analysis.
Independent analyses of parametric images of binding potential (30) that used statistical parametric mapping (SPM 99) were also performed to examine the relation between 5-HT1A receptor binding and explicit memory function at the voxel level (31). Correlation was assessed between the binding potential value at each voxel and the value of the WMS-R general memory index entered as individual covariates of interest. The ligand-specific template image (32) was used to define the stereotactic transformation parameters for the binding potential images of [11C]WAY-100635. Normalized binding potential images were smoothed with a Gaussian filter to 16 mm full-width half-maximum. Significance was thresholded at p<0.001, uncorrected (T=3.50). An extent threshold of 50 contiguous voxels was applied. This threshold is justified if the detected area is known to play an important role in learning and memory.
Tandospirone Administration
Nine healthy male volunteers aged 26–35 years (mean=30.9, SD=3.0) took part in this study. The study was randomized, double-blind, and crossover balanced; all subjects underwent three sessions with at least 7-day intervals, one of which was a placebo session, and two were drug administration sessions that used two different doses. Baseline samples to measure plasma GH were taken just before drug administration at 14:00. Body temperature was recorded by using a digital thermometer accurate to 0.1°C. The subjects then received 30 mg and 60 mg of tandospirone and identical placebo orally in random order on the three occasions. Further blood sampling took place 60 minutes after administration, and then explicit memory was assessed after blood sampling with the Auditory Verbal Learning Test (33). Fifteen words were presented auditorily in the same sequence in five trials, ending with a free recall of the words (immediate recall). After the five trials, an interference list was presented and recalled, and then the subjects were ordered to recall the first list of words (postinterference recall). Twenty minutes later, the subjects were asked to recognize the first list of words (delayed recognition). This widely used test was chosen because it takes a relatively short time and is preferred to other tests under conditions of limited assessment time (34). To exclude any practice effect, three different sets of word lists were presented in random order at the three sessions. A digit span test (raw span forward and backward) and a word fluency test (number of initial letter/semantic category cued words said in 1 minute; these letter and category fluency tests were considered to depend on different underlying neural mechanisms [35]) were also performed. Finally, the subjects completed a computerized Stroop color-naming task (36). Measures of Stroop interference were calculated in milliseconds by subtracting the mean reaction time for incongruent words from that for congruent words. Drug-induced changes of plasma GH and temperature and cognitive variables were examined across conditions by using repeated-measures analysis of variance (ANOVA). Post hoc analysis (Dunnett’s t test) was conducted by comparing the placebo and the two drug conditions; p<0.05 was considered significant.
Results
A significant negative correlation was observed between 5-HT1A receptor binding potential in the hippocampus and the verbal and general memory indices of WMS-R (25, 26) (Table 1 and Figure 1). No significant correlations were observed among any other indices and regions, and there was no age effect on binding potential in any region. The significance of this correlation was consistent with the finding from the analysis using statistical parametric mapping (31), which revealed significant correlation in the bilateral hippocampus (Montreal Neurological Institute coordinates: x=–30, y=–22, z=–14 in the left side; x=34, y=–28, z=–12 and x=40, y=–4, z=–28 in the right side) (Figure 2).
As seen in Table 2, the effect of the stimulation of 5-HT1A receptors with tandospirone on explicit memory function, as measured with the Auditory Verbal Learning Test (33), revealed a significant dose-dependent decline in performance (immediate recall: F=7.33, df=2, 16, p=0.005; postinterference recall: F=4.47, df=2, 16, p<0.03; delayed recognition: F=3.65, df=2, 16, p=0.05). Post hoc analysis revealed that the placebo condition differed significantly from the 60-mg tandospirone condition in immediate recall (t=12.3, df=16, p=0.003), postinterference recall (t=2.22, df=16, p<0.04), and delayed recognition (t=0.67, df=16, p<0.05). On the other hand, there was no significant drug effect on the performance of the word fluency task (initial word fluency: F=2.31, df=2, 16, p=0.13; category word fluency: F=2.22, df=2, 16, p=0.14), digit span (forward: F=0.17, df=2, 16, p=0.85; backward: F=0.26, df=2, 16, p=0.77) or the Stroop task (F=1.37, df=2, 16, p=0.28).
Neuroendocrinological examinations revealed that tandospirone dose-dependently decreased body temperature (F=17.84, df=2, 16, p=0.008) and increased GH (F=12.04, df=1.1, 8.7, p=0.007). Post hoc analysis revealed that the placebo condition differed significantly from the 30- and 60-mg tandospirone conditions regarding change in body temperature (30 mg: t=0.16, df=16, p<0.04; 60 mg: t=0.33, df=16, p=0.0001) and from the 60-mg condition for GH (t=9.0, df=16, p=0.001). The changes in mean outcome parameters of memory function paralleled those of body temperature and GH (Table 2 and Figure 3), although there were no statistically significant correlations among these measures because of their large interindividual variabilities, as indicated by the standard deviations.
Discussion
The present results showed a significant negative correlation between explicit memory function and 5-HT1A receptor binding localized in the bilateral hippocampal area where the postsynaptic 5-HT1A receptors are enriched (3). Although age-related hippocampal atrophy has been reported (37), within the age range of the present study there was no age-related reduction of hippocampal [11C]WAY-100635 binding (r=–0.10, df=14, p>0.70). Furthermore, the present results indicated that decreased [11C]WAY-100635 binding correlated with better memory performance, which was opposite to the reported aging effect. Partial volume effect is unlikely to account for the results presented here.
Since [11C]WAY-100635 binding was not sensitive to either acute or chronic changes of endogenous serotonin (38), our finding might be attributable to the density of 5-HT1A receptors. The results point to an association between high hippocampal 5-HT1A receptor density in subjects and lower explicit memory ability. Furthermore, we found that the administration of tandospirone, a specific 5-HT1A agonist, dose-dependently impaired explicit verbal memory, whereas other cognitive functions showed no significant changes. The change in memory function paralleled those of body temperature and GH, which were reported to be induced by the stimulation of postsynaptic 5-HT1A receptors (23, 24).
When these observations are taken together, it appears evident that the activity of postsynaptic 5-HT1A receptors in the hippocampus has an inhibitory influence on human explicit memory function. Although it was not clear whether individual variability in 5-HT1A receptor densities in the hippocampus is wholly genetically determined or whether it is subject to environmental influences such as stress factors, one could postulate that it is a cause of the individual differences in memory ability.
In animal models, it has been demonstrated that systemic and intrahippocampal injections of 5-HT1A receptor agonists induced memory and learning impairment (39, 40) and that the stimulation of 5-HT1A receptors resulted in neuronal hyperpolarization and inhibition of neuronal activity in the hippocampus (41). Postsynaptic 5-HT1A receptors are predominant on pyramidal neurons (4), and it is reasonable to consider that the negative influence of postsynaptic 5-HT1A receptors on memory function may reflect the decrease in pyramidal cell activity by the inhibitory effect of 5-HT or 5-HT1A agonist on pyramidal neurons in the hippocampus. It was also reported that 5-HT1A antagonists ameliorated the learning and memory impairment induced by muscarinic and NMDA receptor antagonists in animals (42, 43). Cellular localization of 5-HT1A receptors has been demonstrated on both cholinergic and glutamatergic neurons (7, 8), and the antagonism of 5-HT1A receptors may increase their neuronal activity.
On the other hand, in an animal study with 5-HT1A-knockout mice, worsening in learning and memory tests has been reported (44). However, a complete deficit of 5-HT1A receptors may also result in the loss of several neural networks that would be important in control learning and memory (45), and some developmental abnormalities in the formation of neural networks certainly cannot be excluded in knockout mice (46). Chronic administration of tandospirone was reported to improve verbal memory in schizophrenic patients treated with haloperidol (15, 16). However, the lower dose of agonist used in their study might preferentially activate presynaptic 5-HT1A receptors with higher sensitivity located on raphe neurons (47), which provide a feedback regulation of the 5-HT system and decrease the release of 5-HT at postsynaptic sites (48). Atypical antipsychotic drugs that exhibit partial agonism at 5-HT1A receptors may preferentially activate presynaptic 5-HT1A receptors with higher sensitivity while blocking their postsynaptic counterparts (49). Their memory improvement properties appear to be mediated, in part, by their antagonistic effect on postsynaptic 5-HT1A receptors in the hippocampus.
Our finding provides the first documentation of the human in vivo functional molecular mapping of explicit memory that is supported by a neuroendocrinological response. The results show that the postsynaptic 5-HT1A receptors localized in the hippocampal formation have a negative influence on explicit memory function. Our findings give rise to the possibility that the antagonistic effect of postsynaptic 5-HT1A receptors in the hippocampus leads to improvement of human memory function. Drugs that work as antagonists on postsynaptic 5-HT1A receptors may be favorable for improved control of memory impairment.
 |
 |
Received May 29, 2002; revision received Sept. 12, 2002; accepted Sept. 19, 2002. From the Brain Imaging Project, National Institute of Radiological Sciences; CREST Japan Science and Technology Corporation, Saitama, Japan; SHI Accelerator Service Ltd, Tokyo; and Biofunctional Informatics, Graduate School of Allied Health Sciences, Tokyo Medical and Dental University, Tokyo. Address reprint requests to Dr. Suhara, Brain Imaging Project, National Institute of Radiological Sciences, 4-9-1 Anagawa, Inage-ku, Chiba 263-8555, Japan; [email protected] (e-mail). Supported by grants from the Human Frontier Science Program Organization (RG0235/1998-B) and the National Institute of Radiological Sciences. The authors thank C. Halldin and V. Pike for radiotracer preparation; T. Saijo, A. Yamamoto, Y. Asai, S. Ito, and M. Hayashi for their help in data acquisition; T. Nishina and A. Tayama for care of subjects; and Y. Ikejiri for discussions and comments.
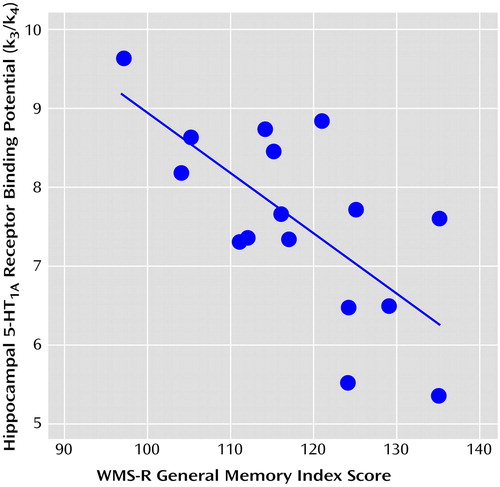
Figure 1. Correlation Between Hippocampal 5-HT1A Receptor Binding Potential and Memory Performance in 16 Healthy Subjectsa
ar=0.69, df=14, p<0.003.
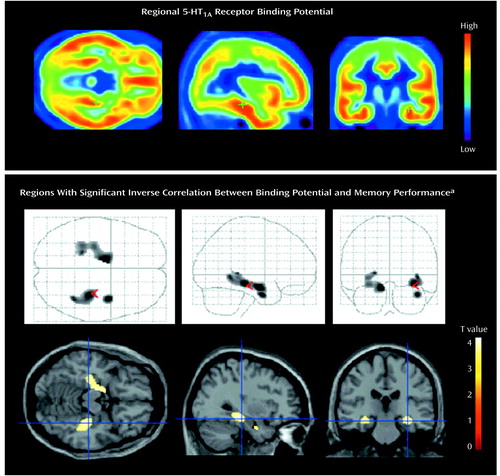
Figure 2. Regional 5-HT1A Receptor Binding Potential in 16 Healthy Subjects and Location of Significant Inverse Correlations Between Binding Potential and Memory Performance
aThe transverse, sagittal, and coronal brain views show voxels with a significant inverse correlation between 5-HT1A receptor binding potential and performance on the General Memory Index from the Wechsler Memory Scale–Revised. Detected areas exceed an uncorrected p value of 0.001 with 50 or more contiguous voxels. These statistical parametric mapping projections were then superimposed on representative transaxial (z=–15), sagittal (x=35), and coronal (y=–25) magnetic resonance images.
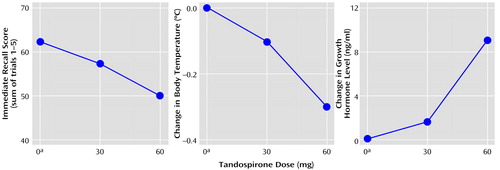
Figure 3. Relation of Tandospirone Dose to Changes in Immediate Recall Performance and Neuroendocrinological Response in 16 Healthy Subjects
aPlacebo.
1. Winkler J, Suhr ST, Gage FH, Thal LJ, Fisher LJ: Essential role of neocortical acetylcholine in spatial memory. Nature 1995; 375:484-487Crossref, Medline, Google Scholar
2. Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ: Genetic enhancement of learning and memory in mice. Nature 1999; 401:63-69Crossref, Medline, Google Scholar
3. Meneses A: 5-HT system and cognition. Neurosci Biobehav Rev 2000; 23:1111-1125Crossref, Google Scholar
4. Buhot MC, Martin S, Segu L: Role of serotonin in memory impairment. Ann Med 2000; 32:210-221Crossref, Medline, Google Scholar
5. Press GA, Amaral DG, Squire LR: Hippocampal abnormalities in amnesic patients revealed by high-resolution magnetic resonance imaging. Nature 1989; 341:54-57Crossref, Medline, Google Scholar
6. Squire LR, Zola-Morgan S: The medial temporal lobe memory system. Science 1991; 253:1380-1386Crossref, Medline, Google Scholar
7. Steckler T, Sahgal A: The role of serotonergic-cholinergic interactions in the mediation of cognitive behaviour. Behav Brain Res 1995; 67:165-199Crossref, Medline, Google Scholar
8. Boast C, Bartolomeo AC, Morris H, Moyer JA: 5HT antagonists attenuate MK801-impaired radial arm maze performance in rats. Neurobiol Learn Mem 1999; 71:259-271Crossref, Medline, Google Scholar
9. Meltzer HY: The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 1999; 21:106S-115SCrossref, Medline, Google Scholar
10. Newman-Tancredi A, Gavaudan S, Conte C, Chaput C, Touzard M, Verriele L, Audinot V, Millan MJ: Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [35S]GTPgammaS binding study. Eur J Pharmacol 1998; 355:245-256Crossref, Medline, Google Scholar
11. Elliott J, Reynolds GP: Agonist-stimulated GTPgamma[35S] binding to 5-HT(1A) receptors in human post-mortem brain. Eur J Pharmacol 1999; 386:313-315Crossref, Medline, Google Scholar
12. Meltzer HY, McGurk SR: The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 1999; 25:233-255Crossref, Medline, Google Scholar
13. Velligan DI, Newcomer J, Pultz J, Csernansky J, Hoff AL, Mahurin R, Miller AL: Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res 2002; 53:239-248Crossref, Medline, Google Scholar
14. McGurk SR: The effects of clozapine on cognitive functioning in schizophrenia. J Clin Psychiatry 1999; 60:24-29Medline, Google Scholar
15. Sumiyoshi T, Matsui M, Nohara S, Yamashita I, Kurachi M, Sumiyoshi C, Jayathilake K, Meltzer HY: Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. Am J Psychiatry 2001; 158:1722-1725Link, Google Scholar
16. Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Kurachi M, Uehara T, Sumiyoshi S, Sumiyoshi C, Meltzer HY: The effect of tandospirone, a serotonin(1A) agonist, on memory function in schizophrenia. Biol Psychiatry 2001; 49:861-868Crossref, Medline, Google Scholar
17. Farde L, Ito H, Swahn CG, Pike VW, Halldin C: Quantitative analyses of carbonyl-carbon-11-WAY-100635 binding to central 5-hydroxytryptamine-1A receptors in man. J Nucl Med 1998; 39:1965-1971Medline, Google Scholar
18. Pike VW, McCarron JA, Lammertsma AA, Osman S, Hume SP, Sargent PA, Bench CJ, Cliffe IA, Fletcher A, Grasby PM: Exquisite delineation of 5-HT1A receptors in human brain with PET and [carbonyl-11 C]WAY-100635. Eur J Pharmacol 1996; 301:R5-R7Google Scholar
19. Shimizu H, Tatsuno T, Hirose A, Tanaka H, Kumasaka Y, Nakamura M: Characterization of the putative anxiolytic SM-3997 recognition sites in rat brain. Life Sci 1988; 42:2419-2427Crossref, Medline, Google Scholar
20. Shimizu H, Karai N, Hirose A, Tatsuno T, Tanaka H, Kumasaka Y, Nakamura M: Interaction of SM-3997 with serotonin receptors in rat brain. Jpn J Pharmacol 1988; 46:311-314Crossref, Medline, Google Scholar
21. Hamik A, Oksenberg D, Fischette C, Peroutka SJ: Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites. Biol Psychiatry 1990; 28:99-109Crossref, Medline, Google Scholar
22. Millan MJ, Rivet JM, Canton H, Le Marouille-Girardon S, Gobert A: Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: a pharmacological characterization of the actions of novel agonists and antagonists. J Pharmacol Exp Ther 1993; 264:1364-1376Medline, Google Scholar
23. Blier P, Seletti B, Young S, Benkelfat C, de Montigny C: Serotonin1A receptor activation and hypothermia: evidence for a postsynaptic mechanism in humans (abstract). Neuropsychopharmacology 1994; 10:92SGoogle Scholar
24. Seletti B, Benkelfat C, Blier P, Annable L, Gilbert F, de Montigny C: Serotonin1A receptor activation by flesinoxan in humans: body temperature and neuroendocrine responses. Neuropsychopharmacology 1995; 13:93-104Crossref, Medline, Google Scholar
25. Wechsler D: Wechsler Memory Scale—Revised. San Antonio, Tex, Harcourt Brace Jovanovich, 1987Google Scholar
26. Sugishita M: The Japanese Version of the Wechsler Memory Scale—Revised. Tokyo, Nihon Bunka Kagakusya, 2001Google Scholar
27. Yasuno F, Hasnine AH, Suhara T, Ichimiya T, Sudo Y, Inoue M, Takano A, Ou T, Ando T, Toyama H: Template-based method for multiple volumes of interest of human brain PET images. Neuroimage 2002; 16:577-586Crossref, Medline, Google Scholar
28. Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C: PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 1999; 46:1375-1387Crossref, Medline, Google Scholar
29. Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, Hume SP, Grasby PM, Lammertsma AA: Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. Neuroimage 1998; 8:426-440Crossref, Medline, Google Scholar
30. Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ: Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997; 6:279-287Crossref, Medline, Google Scholar
31. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapp 1995; 2:189-210Crossref, Google Scholar
32. Meyer JH, Gunn RN, Myers R, Grasby PM: Assessment of spatial normalization of PET ligand images using ligand-specific templates. Neuroimage 1999; 9:545-553Crossref, Medline, Google Scholar
33. Rey A: L’examen clinique en psychologie. Paris, Presses Universitaires de France, 1964Google Scholar
34. Mitrushina M, Satz P, Chervinsky A, D’Elia L: Performance of four age groups of normal elderly on the Rey Auditory-Verbal Learning Test. J Clin Psychol 1991; 47:351-357Crossref, Medline, Google Scholar
35. Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, Van Horn JD, Berman KF: A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology 2000; 14:353-360Crossref, Medline, Google Scholar
36. Ilan AB, Polich J: Tobacco smoking and event-related brain potentials in a Stroop task. Int J Psychophysiol 2001; 40:109-118Crossref, Medline, Google Scholar
37. Pruessner JC, Collins DL, Pruessner M, Evans AC: Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci 2001; 21:194-200Crossref, Medline, Google Scholar
38. Maeda J, Suhara T, Ogawa M, Okauchi T, Kawabe K, Zhang MR, Semba J, Suzuki K: In vivo binding properties of [carbonyl-11C]WAY-100635: effect of endogenous serotonin. Synapse 2001; 40:122-129Crossref, Medline, Google Scholar
39. Carli M, Tatarczynska E, Cervo L, Samanin R: Stimulation of hippocampal 5-HT1A receptors causes amnesia and anxiolytic-like but not antidepressant-like effects in the rat. Eur J Pharmacol 1993; 234:215-221Crossref, Medline, Google Scholar
40. Kant GJ, Meininger GR, Maughan KR, Wright WL, Robinson TN III, Neely TM: Effects of the serotonin receptor agonists 8-OH-DPAT and TFMPP on learning as assessed using a novel water maze. Pharmacol Biochem Behav 1996; 53:385-390Crossref, Medline, Google Scholar
41. Pugliese AM, Passani MB, Corradetti R: Effect of the selective 5-HT1A receptor antagonist WAY 100635 on the inhibition of epsps produced by 5-HT in the CA1 region of rat hippocampal slices. Br J Pharmacol 1998; 124:93-100Crossref, Medline, Google Scholar
42. Carli M, Bonalumi P, Samanin R: WAY 100635, a 5-HT1A receptor antagonist, prevents the impairment of spatial learning caused by intrahippocampal administration of scopolamine or 7-chloro-kynurenic acid. Brain Res 1997; 774:167-174Crossref, Medline, Google Scholar
43. Harder JA, Ridley RM: The 5-HT1A antagonist, WAY 100635, alleviates cognitive impairments induced by dizocilpine (MK-801) in monkeys. Neuropharmacology 2000; 39:547-552Crossref, Medline, Google Scholar
44. Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M: Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin (1A) receptors. Proc Natl Acad Sci USA 2000; 97:14731-14736Crossref, Medline, Google Scholar
45. Millan MJ, Lejeune F, Gobert A: Reciprocal autoreceptor and heteroreceptor control of serotonergic, dopaminergic and noradrenergic transmission in the frontal cortex: relevance to the actions of antidepressant agents. J Psychopharmacol 2000; 14:114-138Crossref, Medline, Google Scholar
46. Whitaker-Azmitia PM: Role of serotonin and other neurotransmitter receptors in brain development: basis for developmental pharmacology. Pharmacol Rev 1991; 43:553-561Medline, Google Scholar
47. Meller E, Goldstein M, Bohmaker K: Receptor reserve for 5-hydroxytryptamine1A-mediated inhibition of serotonin synthesis: possible relationship to anxiolytic properties of 5-hydroxytryptamine1A agonists. Mol Pharmacol 1990; 37:231-237Medline, Google Scholar
48. Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C: Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann NY Acad Sci 1998; 861:204-216Crossref, Medline, Google Scholar
49. Millan MJ: Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther 2000; 295:853-861Medline, Google Scholar



