PET Studies of the Influences of Nicotine on Neural Systems in Cigarette Smokers
Abstract
OBJECTIVE: The effects of acute nicotine administration and smoking on brain function were investigated in two studies, with the primary goal of identifying neural systems that mediate these effects. METHOD: In study 1, 18 healthy volunteer cigarette smokers were exposed to three conditions in a single session: 1) smoking a nicotine-containing cigarette, 2) smoking a denicotinized cigarette, or 3) receiving intravenous nicotine injections in conjunction with smoking a denicotinized cigarette. In study 2, 16 subjects smoked a nicotine-containing and denicotinized cigarette in each of two sessions 2 hours after receiving the nicotinic antagonist mecamylamine (10 mg) or placebo orally. Regional cerebral blood flow (rCBF) was assessed by using the bolus 15O-labeled water method and positron emission tomography. Subjective measures of smoking withdrawal symptoms were also collected. RESULTS: A principal-components analysis of rCBF data pooled from the two studies identified three factors consisting of frontal, striatal, and reticular systems. The amygdala was considered as a separate region of interest. Nicotine increased normalized rCBF in the left frontal region and decreased rCBF in the left amygdala. The rCBF in the right hemisphere reticular system was related to nicotine dose in an inverted-U-shaped pattern and was strongly related to self-reported craving for cigarettes and to the addiction scale of a smoking motivation questionnaire. The effects of mecamylamine on rCBF were generally opposite to those of nicotine. CONCLUSIONS: The results indicate that nicotine influences brain regions involved in arousal and reward and suggest specific functional systems that may be linked to motivationally significant aspects of tobacco dependence.
Cigarette smoking continues to be a widespread public health problem; of the nearly 1 billion smokers worldwide, half are likely to die of smoking-related diseases (1, 2). The maintenance of smoking behavior is believed to hinge on the addictive effects of nicotine (3), mediated predominantly through the actions of the drug at nicotinic acetylcholine receptors (4), which are distributed throughout many cortical and subcortical brain regions, notably the thalamus and midbrain (5). Through actions at these receptors, nicotine has widespread effects on the central and peripheral nervous systems. These effects include facilitation of neurotransmitter release from dopaminergic, cholinergic, glutamatergic, serotonergic, and γ-aminobutyric acid-ergic nerve terminals (6–8). Nicotine has cognitive-enhancing, attention-gating, stress-alleviating, and weight-regulating effects that may contribute to tobacco dependence (9–13).
Studies of acute effects of nicotine on regional cerebral metabolic rates for glucose in rats have shown marked stimulation that was blocked by mecamylamine (14–16). Although studies in animals have provided important clues, the effects that are most critical in maintaining nicotine dependence in humans remain a puzzle. Brain imaging, however, offers the hope of elucidating the reinforcing effects of nicotine in smokers, thereby improving our understanding of tobacco addiction.
A preliminary study of the effect of intravenous nicotine (1.5 mg) on regional cerebral metabolic rates for glucose in five human subjects was conducted with positron emission tomography (PET) (17). Unlike results obtained in rats, the results in the human subjects suggested that nicotine reduced cerebral glucose metabolism by about 10%. Nicotine administration by means of nasal spray also caused a small reduction in global glucose metabolism, but an analysis of normalized measures showed relative activation in the left posterior cingulate gyrus, left lateral occipitotemporal gyrus, right hemisphere thalamus, and visual cortex. Decreases in normalized metabolic rate were detected in the left insula and right inferior occipital gyrus (18).
In addition to studies of brain metabolic activity, which have relatively poor time resolution, cerebral blood flow measurements can be used to assess transient central nervous system actions of nicotine and/or smoking. For example, Mathew and Wilson (19) found that cigarette smoking significantly increased hemispheric blood flow as assessed by transcranial Doppler ultrasonography. The effects of nicotine on regional cerebral blood flow (rCBF) have also been studied using 15O-labeled water and PET. Using this method, Zubieta et al. (20) described acute effects of nasal nicotine administration on rCBF. Nicotine increased rCBF in the thalamus, and reductions were noted in the amygdala and temporal cortex. Other studies have found increases in CBF after nicotine administration (e.g., references 21–23). Nagata et al. (24) reported significant increases in rCBF after cigarette smoking, most notably in the frontal lobes and cerebellum. Stein et al. (25), using functional magnetic resonance imaging (fMRI) to assess rCBF, reported that rapid intravenous nicotine administration induced widespread activation in several brain regions, including the amygdala, cingulate cortex, thalamus, basal ganglia, and frontal lobes. However, different results have been obtained in other studies, including no effect or decreases in CBF (26–28).
Methodological factors related to nicotine dosing might have contributed to the variable effects of nicotine reported. In this regard, most studies have not adequately controlled for the potential effects of the nonnicotine components of cigarette smoke. Previous studies have also not always used naturalistic nicotine dosing parameters, in some cases requiring smokers to puff much more rapidly than they are accustomed to doing or using cigarettes that deliver much more nicotine than is typically delivered.
The present report details two studies that used PET to characterize the acute effects of nicotine on rCBF of cigarette smokers, with rigorous control of nicotine dosing. Concurrently, subjective measures of the rewarding effects of nicotine were obtained to link the actions of nicotine on rCBF to the subjective effects that may reflect reinforcing effects.
General Method
Individualizing and Controlling Nicotine Dose
Denicotinized cigarettes
We used denicotinized cigarettes (Philip Morris, Inc., Richmond, Va.), whose taste and tar delivery (9 mg when smoked, according to Federal Trade Commission [FTC] criteria) were similar to those of nicotine-containing cigarettes. The nicotine delivery is extremely low, less than 0.1 mg (29), and measurements of arterial and venous plasma nicotine concentrations from subjects in the present study (reported elsewhere [30]) indicated that the denicotinized cigarettes produced no significant boosts in plasma nicotine levels.
Controlled smoke delivery apparatus
Puff volume was controlled with a simple apparatus validated in previous studies (31). The device used a glass syringe preloaded with a measured amount of air that was supplied to the burning cigarette with each puff.
Individualized nicotine dosing
Because there is enormous variability between cigarette smokers in the nicotine dose extracted from cigarettes during ad lib smoking (32), the number of puffs, puff volumes, and interpuff intervals (for both nicotine and denicotinized cigarettes) were individualized to match the characteristic smoking pattern of each participant. This was accomplished by measuring smoking characteristics at baseline by using the smoke delivery apparatus described in the previous section. The average dose of nicotine per puff was calculated by reproducing each subject’s smoking pattern and analyzing the residue trapped in Cambridge filters (33). To minimize variability in nicotine absorption from variations in inhalation, subjects in all conditions were instructed to inhale deeply and hold their breath for 5 seconds after each puff (34).
In study 1, one of the experimental conditions included intravenous administration of nicotine. Each puff-sized nicotine dose was delivered over approximately 1 second in 2 ml sterile saline injected directly into a catheter (30); the total dose equaled that obtained from the cigarette in the ad lib smoking assessment.
PET and MRI Procedures
CBF imaging protocol
PET imaging was performed on a General Electric Medical Systems Advance scanner (35) (Milwaukee). To measure rCBF with PET, we implemented the [15O]water autoradiographic technique as described by Meyer (36). After an intravenous bolus injection of 10 mCi of [15O]water, each three-dimensional PET measurement acquisition began when a total count rate of 100,000 counts/second was reached and continued for 60 seconds. In study 1, absolute quantitation was performed; an intra-arterial catheter was placed in the radial artery, and the arterial input function (20 2-ml samples during the first 3 minutes) was obtained and corrected for decay. The tomograph and well counter were calibrated daily for the quantitative studies. Data were corrected for random events, scatter, detector normalization, and attenuation (see below) before reconstruction by means of filtered back-projection. For images to be used in the region-of-interest analysis, only a ramp filter was used to minimize partial volume effects. Images were reconstructed into 128×128×35 image sets with 2-mm pixels. Each subject’s head was aligned in a plane roughly parallel to the glabella-inion line. A transmission scan was done before or after each emission scan for the purpose of attenuation correction.
MRI
Transaxial images were acquired with a 1.5-T Signa scanner (GE Medical Systems, Milwaukee). Both T2-weighted (TR=2500 msec, TE=20 msec and TE=80 msec, number of excitations=1) and T1-weighted (TR=600 msec, TE=20 msec, number of excitations=2) images were acquired with a 128×256 matrix and a 24-cm field of view. Contiguous 3-mm slices were acquired for coregistration with the PET images.
Image registration
We registered the PET image for each subject to his/her own MRI using the methods of Pelizzari et al. (37) with modifications (38). The image registration allows reorienting of the PET images by reslicing to match the MR images within 1–2 mm accuracy (38) so that MRI-identified brain regions can be applied to the PET data. T1-weighted images were used for determination of regions of interest. Region-of-interest evaluation started with the sampling of brain regions on each subject’s MR image. A trained operator drew regions that included the appropriate gray matter.
Nonspecific factors and their control
Subjects were asked to avoid alcohol and caffeine as well as nicotine for 8 hours before the scanning session. A mock PET scan was performed without radiopharmaceutical administration to facilitate habituation to the procedure. Subjects were instructed to stay awake and were allowed to read during the period between smoking conditions.
Smoking-Related Dependent Measures
Smoking withdrawal symptoms
We used a withdrawal questionnaire based on that of Shiffman and Jarvik (39, 40) to assess craving, negative affect, arousal, somatic symptoms, and appetite.
Smoking motivation questionnaires
A questionnaire based on that of Ikard et al. (41), which assessed self-reported motivations for smoking, was administered. The items were scored on scales for stimulation, handling, relaxation, tension reduction, addiction (craving reduction), habit, and psychosocial motives for smoking.
The Fagerström Test for Nicotine Dependence questionnaire (42) was also administered.
Smoking behavior
Subjects’ expired air carbon monoxide (CO) concentrations were measured by using a handheld CO monitor (Vitalograph, Lenexa, Kan.). Expired air CO concentration was calculated by subtracting the background (ambient) CO from the peak CO reading.
Data Analysis
Data analysis was performed by using SuperANOVA (Abacus Concepts, Berkeley, Calif.) and StatView (SAS Institute, Cary, N.C.). These software packages apply a multivariate approach to repeated measures analysis that takes into account the correlation pattern among repeated measurements (43). Before conducting analyses of variance (ANOVAs) and analyses of covariance (ANCOVAs) contrasting the experimental conditions, a principal-components analysis was conducted to identify combinations of variables reflecting effects of nicotine on rCBF. This approach had the advantages of 1) limiting type I error by reducing the large number of regions of interest to relatively few dependent variables, 2) reducing type II error relative to other multivariate approaches (44), and 3) identifying functional brain systems that mediate nicotine’s effects. On the basis of previous animal and human research, data from the following brain regions of interest were entered into the factor analysis: anterior cingulate, posterior cingulate, prefrontal cortex, occipital cortex, caudate nucleus, putamen, ventral striatum, amygdala, septal nucleus, hippocampus, thalamus, midbrain (excluding the colliculi), and pons. Normalized rCBF was calculated by dividing the radioactivity in each region by the average radioactivity in the entire left and right cerebral hemisphere gray matter.
Comparable data from study 1 and study 2 were pooled by concatenating the raw data sets to determine a common set of dependent measures. Since both studies included a postsmoking assessment of rCBF after subjects smoked their usual brand of nicotine-containing cigarette and the denicotinized cigarette, the difference in rCBF between these two conditions was entered as the raw data into the analysis. A scree plot, which indicates when substantially less variance is accounted for with successive factors (45), was used to identify three factors with substantial eigenvalues (>1.5). The factors and associated loadings for the oblique solution are presented in Table 1. Intercorrelations between the factors were r=–0.09 for factor 1 versus factor 2, r=0.06 for factor 1 versus factor 3, and r=–0.01 for factor 2 versus factor 3. Loadings with a magnitude of at least 0.5 on a given factor were taken to be large, and the corresponding brain regions were selected to define factor scores. These factor scores were computed as the average of standardized variables (mean=0, SD=1) on the basis of the normalized rCBF values for the selected regions, as follows: factor 1 (“striatal”)—caudate, putamen, and posterior cingulate (negative loading); factor 2 (“reticular”)—pons, midbrain, thalamus, and occipital cortex; and factor 3 (“frontal”)—ventral striatum, prefrontal cortex, anterior cingulate, and occipital cortex (negative loading). Data for the amygdala were analyzed separately, as this region did not load strongly on any factor but was of interest because of its role in emotion and reward evaluation (46).
Thus, four dependent measures assessing rCBF immediately after each cigarette condition were analyzed by ANOVA and ANCOVA in each of the studies. In study 1, repeated measures analyses with hemisphere (left or right) and cigarette condition (nicotine-containing cigarette or denicotinized cigarette) as factors were conducted; in study 2, hemisphere, cigarette condition, and mecamylamine condition (mecamylamine or placebo) were included as factors in the analyses. Analyses were conducted both with and without nicotine dose as a covariate, including linear (on its own) and quadratic (controlling for linear) terms. When significant interactions of nicotine dose with one or more of the other factors were detected, they were analyzed in follow-up tests. These tests assessed the differences between the nicotine and denicotinized cigarette conditions as a function of dose at the different levels of the other factors (e.g., hemisphere or mecamylamine condition) (47). The alpha criterion for assessing drug effects was 0.05 (two-tailed). For study 1, in which absolute quantification of cerebral blood flow was performed, the postsmoking change in perfusion was also analyzed as a function of hemisphere, nicotine dose, and cigarette condition.
To identify brain regions that might be important to nicotine dependence, effects of nicotine on withdrawal symptoms were correlated with the effects of nicotine on the factors assessing rCBF by using data from both studies.
Study 1
The experiment exposed subjects to three conditions in a crossover design: 1) usual brand of nicotine-containing cigarette smoke accompanied by intravenous saline infusion, 2) denicotinized cigarette smoke accompanied by intravenous saline infusion), and 3) intravenous nicotine infusion accompanied by denicotinized cigarette smoke. In conditions 1 and 3, the nicotine dose was individualized to duplicate as closely as possible the effects to which each participant was accustomed. The use of denicotinized cigarettes allowed for partial control of nonnicotine aspects of smoking behavior and smoke constituents, such as tar, CO, and CO2, that might affect cerebral perfusion.
Subjects
Eighteen healthy volunteers were recruited from the community by means of newspaper advertisements. To facilitate subject recruitment, volunteers were provided with two incentives: 1) they were paid $150 per session and 2) they were offered the opportunity to participate in a smoking cessation treatment trial. To be included in the study, subjects had to be 21–55 years of age and right-hand dominant, to smoke at least 20 cigarettes/day of a brand delivering at least 0.5 mg of nicotine when smoked according to the standard FTC method (48), and to have an expired CO concentration (measured in the afternoon during the screening for study participation) of >15 ppm. Subjects were excluded if they showed clinically significant abnormalities on the basis of the results of a physical examination, ECG, serum chemistry measures, CBC, and urinalysis. After a complete description of the study, written informed consent was obtained from each participant.
Procedure
Subjects participated in a single morning session of approximately 5 hours’ duration, conducted after overnight abstinence from cigarettes. In the denicotinized smoke conditions, the same numbers of puffs, puff volume, and interpuff intervals were used as in the nicotine-containing smoke condition. The order of conditions was counterbalanced across subjects. Carryover effects were minimized by allowing 60-minute intervals between successive test conditions; previously we found that plasma nicotine levels and subjective effects after a single dose of nicotine dissipate within 60 minutes.
In addition to arterial sampling for quantitation of rCBF, venous blood samples (5 cc) were drawn after each cigarette/infusion condition for nicotine assay by using gas chromatography (49). Subjects’ heart rate and blood pressure were measured immediately before and after each condition. Subjects also completed a smoking withdrawal symptom questionnaire and a smoking evaluation questionnaire after each condition.
Before the PET scan session, each subject underwent an MRI scan to allow for anatomical region-of-interest localization (as described earlier). Subjects also completed a baseline smoking session, conducted after overnight abstinence, in which ad lib nicotine self-administration was measured according to procedures described earlier. Each subject’s measurement of ad lib nicotine self-administration was used in selecting the dose of controlled nicotine administered to the subject.
Data Analysis
Owing to problems with insertion or maintaining patency of the arterial line, absolute quantification of blood flow results was obtained for only 10 of the 18 subjects. Factor scores based on normalized rCBF were available for 17 of the 18 subjects (one subject had dental appliances that interfered with the MRI, precluding definition of regions of interest). Normalized flow was highly correlated with absolute flow in regions for which data for both calculations were available.
Postsmoking values of normalized rCBF were calculated on the basis of the factors described earlier. When the effects of the cigarette condition were significant, these effects were analyzed further with the following two contrasts: 1) to assess the effects of nicotine per se, the effects of denicotinized cigarette smoke accompanied by nicotine infusion were compared to the effects of denicotinized cigarette smoke accompanied by saline infusion; and 2) to assess the overall effects of the usual brand of cigarette, which would include any unique effects of inhaled nicotine, the effects of the usual brand of nicotine-containing cigarette smoke accompanied by saline infusion were compared to the effects of the denicotinized cigarette smoke accompanied by saline infusion.
Results
Subjects
Fourteen men and four women participated in the study; the mean age was 39.1 years (SD=9.0), and subjects had smoked for an average of 19.9 years (SD=9.6). Subjects smoked a mean of 28 cigarettes/day (SD=10) with a mean nicotine delivery (by FTC method) of 0.77 mg (SD=0.15) per cigarette. The subjects’ mean score on the Fagerström Test for Nicotine Dependence questionnaire was 7.1 (SD=1.6), indicating moderately high nicotine dependence.
Nicotine dose and plasma levels
Based on each subject’s ad lib smoke intake, the mean dose of nicotine administered to the subjects was 0.74 mg (SD=0.32, range=0.30–1.60) both in the nicotine-containing cigarette/saline infusion condition and in the denicotinized cigarette/nicotine infusion condition. Plasma nicotine levels were assessed in arterial blood samples collected immediately before and after smoking for nine subjects, and in venous samples collected after smoking for 15 subjects. Before smoking, nicotine levels were quite low, consistent with overnight abstinence (mean=1.5 ng/ml, SD=1.0). As intended, the nicotine levels achieved after controlled doses of smoke from the usual brand of nicotine-containing cigarette were nearly identical to those after intravenous nicotine administration: on average, arterial nicotine increased by 20.5 ng/ml (SD=9.7) and 20.4 ng/ml (SD=9.6), respectively (F=0.00, df=1, 8, p=0.97); postsmoking and postinfusion nicotine levels in venous blood were 9.3 ng/ml (SD=6.7) and 11.9 ng/ml (SD=9.0), respectively (F=2.76, df=1, 14, p=0.12). In the denicotinized cigarette/saline infusion condition, the change in arterial nicotine level was very low (1.6 ng/ml, SD=1.4). Postsmoking venous nicotine levels, reflecting not only any immediate nicotine intake but also potential carryover from previous conditions, were also very low (mean=4.1, SD=2.4). The difference in nicotine levels between the two denicotinized cigarette/infusion conditions (the denicotinized cigarette/nicotine infusion condition versus the denicotinized cigarette/saline infusion condition) was highly significant (F=35.30, df=1, 16, p=0.0001 for arterial nicotine change; F=17.47, df=1, 28, p=0.0003, for postsmoking venous nicotine level).
Effect of nicotine on global blood flow
Absolute blood flow for the left and right hemisphere gray matter was analyzed for the effects of the cigarette/infusion conditions. The contrast between the two denicotinized cigarette/infusion conditions showed an interactive effect of hemisphere (left versus right), condition (denicotinized cigarette/nicotine infusion versus denicotinized cigarette/saline infusion), and dose (F=6.99, df=1, 8, p=0.03). The interaction resulted from different responses of the left versus the right hemisphere blood flow to the intravenous nicotine, compared with saline. Specifically, the difference in the nicotine effect between the left and right hemispheres was negatively correlated with nicotine dose (r=–0.68, p=0.03, N=10) (Figure 1). This interaction was not attributable to one or more simple effects relating dose to perfusion in each hemisphere and each condition. The difference between the effects of nicotine-containing cigarette smoke accompanied by saline infusion and the effects of denicotinized cigarette smoke accompanied by saline infusion tended to parallel the difference between the effects of the nicotine and saline infusions but was not significant. The mean postsmoking changes in perfusion (cm3/minute per 100 g tissue) were –2.5 (SD=5.4) in the left hemisphere and –3.2 (SD=6.1) in the right hemisphere for the nicotine-containing cigarette/saline infusion condition, 0.4 (SD=6.1) in the left and 0.1 (SD=5.9) in the right for the denicotinized cigarette/saline infusion condition, and –4.3 (SD=7.8) in the left and –5.0 (SD=7.7) in the right for the denicotinized cigarette/nicotine infusion condition. Thus, nicotine tended to reduce global blood flow, more in the right hemisphere than in the left, and more when administered by the intravenous dose than when given in a cigarette.
Normalized blood flow
The normalized blood flow data for study 1 were analyzed in terms of the factors derived from the pooled data of studies 1 and 2, as described in the General Method section.
Reticular factor
A significant interaction emerged between hemisphere and dose (quadratic term) (F=8.99, df=1, 14, p<0.01), and there was an interaction of cigarette condition and dose (quadratic term) (F=3.79, df=2, 28, p=0.04). These interactions were analyzed in terms of simple effects by conducting two regression analyses against dose: 1) for the normalized blood flow differences between the nicotine-containing cigarette/saline infusion condition and the denicotinized cigarette/saline infusion condition (reflecting the nicotine effect of the cigarette) and 2) for the normalized blood flow differences between the denicotinized cigarette/nicotine infusion condition and the denicotinized cigarette/saline infusion condition (reflecting the effect of intravenous nicotine). For both hemispheres, the data for the relation of the nicotine dose with the difference in score between two cigarette/saline infusion conditions fit an inverted-U-shaped curve (F=4.57, df=1, 14, p=0.05 and F=2.08, df=1, 14, p=0.03 for the quadratic term for the left and right hemispheres, respectively). The contrast between the two denicotinized cigarette/infusion conditions showed a nonsignificant inverted-U relationship for the right hemisphere only (F=3.23, df=1, 14, p=0.10, for the quadratic dose term).
Frontal factor
There was a significant cigarette condition-by-dose (quadratic term) interaction (F=9.43, df=2, 28, p=0.0007). An analysis of simple effects showed that, averaged across both hemispheres, the difference in normalized blood flow between the nicotine-containing cigarette/saline infusion condition and the denicotinized cigarette/saline infusion condition showed a U-shaped dose-related increase (F=14.40, df=1, 14, p=0.002). The contrast between the denicotinized cigarette/nicotine infusion condition and the denicotinized cigarette/saline infusion condition showed no significant relationship with nicotine dose.
Amygdala
Normalized rCBF for the amygdala showed an interaction of hemisphere, cigarette condition, and dose (F=4.16, df=2, 30, p=0.03). An analysis of simple effects by hemisphere and condition showed that this interaction reflected a U-shaped dose-response relation, for the amygdala in the right hemisphere, for the difference between the nicotine-containing cigarette/saline infusion condition and the denicotinized cigarette/saline infusion condition (F=5.42, df=1, 14, p=0.04 for the quadratic term as a function of nicotine dose). The contrast between the denicotinized cigarette/nicotine infusion condition and the denicotinized cigarette/saline infusion condition showed a similar but nonsignificant relationship (p>0.1).
Striatal factor
No significant effects of cigarette condition or nicotine were detected for this factor.
Study 2
The experiment comprised a two-by-two (nicotine-containing cigarette versus denicotinized cigarette and mecamylamine versus placebo [no mecamylamine]) repeated measures design. The goals were to detect the effects of nicotine on rCBF and to determine whether these effects were susceptible to blockade by the nicotinic antagonist mecamylamine.
Subjects
Sixteen subjects were recruited for the study. The subject selection criteria were identical to those of study 1, with the exception of the nicotine intake criterion. Since some subjects in study 1 self-administered relatively low doses of nicotine during their baseline ad lib smoking assessment, for study 2 we imposed the additional criterion that only subjects who inhaled at least 0.6 mg of nicotine from the cigarette during the baseline assessment would be included. After a complete description of the study, written informed consent was obtained from each participant.
Procedure
Two morning sessions of approximately 3 hours’ duration were conducted after overnight abstinence from cigarettes. Two hours before each session, subjects swallowed a capsule containing 10 mg of mecamylamine hydrochloride or placebo. In each session, PET was used to assess the acute rCBF effects of either 1) controlled doses of nicotine-containing smoke, matching the dose to that self-administered in a 10-minute ad lib smoking period during a baseline session; or 2) puffs of denicotinized smoke (same numbers of puffs, puff volume, and interpuff intervals as were used in the nicotine-containing smoke condition). The order of conditions was counterbalanced across subjects. Carryover effects were minimized by allowing 60-minute intervals between conditions. PET scanning immediately followed the smoking of each cigarette (four scans over two sessions). Only normalized blood flow measures (without arterial blood sampling) were collected in this study.
Data Analysis
Normalized blood flow data were analyzed by using the same factors as were used in study 1, with cigarette (nicotine versus denicotinized), mecamylamine condition, and dose as independent variables in the model. Data from one subject were excluded owing to an insufficient radioactivity count rate after the bolus [15O]water injection.
Results
Subjects
Five men and 11 women participated in the study; the mean age was 39.2 years (SD=10.5), and subjects had smoked for an average of 23.6 years (SD=17.2). Subjects smoked an average of 21.0 cigarettes/day (SD=9.1), with a mean nicotine delivery (by FTC method) of 1.0 mg (SD=0.2). Their mean score on the Fagerström Test for Nicotine Dependence questionnaire was 5.9 (SD=1.8), indicating moderate nicotine dependence. The mean self-administered dose of nicotine during the smoking baseline assessment was 1.1 mg (SD=0.5, range=0.6–2.5).
Normalized blood flow
Reticular factor
With nicotine dose included as a covariate, ANCOVA showed interactive effects of nicotine dose with cigarette condition, mecamylamine condition, and hemisphere (F=8.84, df=1, 13, p=0.01). Follow-up analyses were conducted as in study 1 by using the difference scores between the nicotine-containing and denicotinized cigarette conditions as the dependent measure (with mecamylamine and no-mecamylamine conditions analyzed separately). These analyses showed that, in the no-mecamylamine conditions, the nicotine effect was positively related to nicotine dose in the left hemisphere and negatively related to dose in the right hemisphere (F=6.36, df=1, 13, r=0.57 p=0.03 for the left hemisphere; F=4.02, df=1, 13, r=–0.49, p=0.07 for the right hemisphere). These relationships were abolished in the mecamylamine conditions.
Frontal factor
There was a main effect for mecamylamine to increase frontal blood flow (F=11.14, df=1, 14, p=0.005); the mean value of the frontal factor score was 0.209 (SD=0.588) in the mecamylamine condition and –0.126 (SD=0.568) in the no-mecamylamine condition. In addition, there was a hemisphere-by-cigarette interaction (F=9.62, df=1, 14, p=0.008). An analysis of simple effects showed that this interaction was accounted for by a right-hemisphere decrease in frontal blood flow in the two nicotine-containing cigarette conditions (both with and without mecamylamine) (factor score mean=–0.064, SD=0.671) relative to the two denicotinized cigarette conditions (both with and without mecamylamine) (factor score mean=0.132, SD=0.586) (F=9.75, df=1, 14, p=0.008).
Amygdala
Without dose as a covariate, there was an overall main effect of mecamylamine (F=7.16, df=1, 14, p=0.02), with mecamylamine increasing normalized blood flow (mean=0.977, SD=0.044, for mecamylamine, and mean=0.944, SD=0.062, for no mecamylamine, respectively). However, as in study 1, a significant interaction of hemisphere, cigarette, and dose was detected (F=5.78, df=1, 13, p=0.03). In terms of simple effects, there was a significant negative linear relation between nicotine dose and the left hemisphere difference between the two nicotine-containing cigarette conditions (both with and without mecamylamine) and the two denicotinized cigarette conditions (both with and without mecamylamine) (F=7.46, df=1, 13, r=–0.6, p=0.02).
Striatal factor
No significant effects were detected for this factor.
Integration of Results From the Two Studies
Nicotine Effects on Normalized rCBF Factors
To identify commonalities in nicotine effects across the two studies, the data were subjected to additional analyses, pooling measures from both studies that reflected the differences in scores between the nicotine cigarette and denicotinized cigarette conditions for the four blood-flow factors. For study 1, the differences between the nicotine-containing cigarette/saline infusion condition and the denicotinized cigarette/saline infusion condition served as the dependent variables. For study 2, the differences between the nicotine-containing cigarette condition and the denicotinized cigarette condition were also used. (For the reticular and striatal factors, which showed interactions of mecamylamine with dose, the difference between the effects of the nicotine-containing cigarette and the denicotinized cigarette in the no-mecamylamine condition was used; for the amygdala and frontal factor, where there were no interactions with mecamylamine, the averages for the mecamylamine and no-mecamylamine conditions were used).
Hemisphere, dose, and study were included as independent variables in the multiple regression model, and backward elimination was performed, with significant interaction terms retained. A higher-order term was only considered if its component lower-order effects were included in the model.
The reticular factor showed a significant interaction between hemisphere and dose (F=8.00, df=1, 30, p=0.008, for the linear dose term; F=7.75, df=1, 29, p=0.009, for the quadratic dose term). Figure 2 depicts the relationship between the reticular blood flow factor and nicotine dose. The left hemisphere normalized blood flow tended to increase with dose, while the right hemisphere blood flow tended to decrease.
The amygdala showed an interaction of hemisphere and dose (F=4.20, df=1, 30, p=0.05). This interaction was due to a relationship between nicotine effect and dose in the left hemisphere (F=4.54, df=1, 30, r=–0.4, p=0.04) (Figure 3).
The frontal factor evidenced a significant interaction between study and nicotine dose effects, and hence the different pattern of results for study 1 and study 2 could not be reduced to a simple overall finding. Study 1 found a U-shaped dose-related increase in normalized blood flow in both hemispheres, whereas study 2 found only a decrease in the right hemisphere.
The striatal factor showed no significant effects of nicotine or dose from the pooled analysis.
Correlations Between Normalized rCBF and Subjective Measures
To explore potential relationships between normalized rCBF and subjective responses to nicotine, the difference scores in normalized blood flow for the nicotine-containing cigarette condition compared with the denicotinized cigarette condition were correlated with the corresponding subjective measures collected in both studies.
A strong positive association was found between the nicotine effect on craving for cigarettes and normalized blood flow for the right hemisphere reticular factor (F=10.02, df=1, 30, r=0.5, p=0.003) (Figure 4). The addiction subscale of the smoking motivation questionnaire also showed a positive correlation with the right-left hemisphere difference in the reticular factor (F=6.42, df=1, 30, p=0.02).
Discussion
Nicotine affected blood flow differentially in specific brain regions. Consistently across studies, nicotine delivery was associated with a dose-related effect on normalized blood flow within the reticular system. The brain regions included in this factor, such as the pons, midbrain, and thalamus, contain high densities of nicotinic receptors (5, 50, 51), and previous brain imaging studies of nicotine effects have found activation of these regions. For example, intranasal nicotine produced activation of the thalamus in PET studies (18, 20), and rapid intravenous nicotine dosing produced activation of several regions in the reticular arousal system in an fMRI study (25). The present results extend these findings, showing that typical doses and rates of nicotine administration produce these changes in rCBF. In addition, our results suggest a biphasic relationship between reticular activation and nicotine dose, with higher doses decreasing reticular activation. The latter may reflect desensitization of nicotinic receptors after their initial activation (52, 53); however, a series of measurements of blood flow over time would be needed to assess this possibility.
Our findings are concordant with previous work suggesting a biphasic dose-response relation for nicotine and arousal, with low doses having a stimulant effect and higher doses a sedative influence (10, 54). Ashton et al. (55), for example, reported a biphasic dose-effect relation for the effect of nicotine on the contingent negative variation (CNV), an EEG measure of cortical arousal. Dunn (56) summarized several studies suggesting that nicotine might counteract the disruptive effects of reticular activating system activity on task performance.
Blood flow in the right hemisphere reticular system showed not only dose-related effects of nicotine but also a high correlation with subjective measures of craving for cigarettes. The results are in accord with neurophysiological studies in the rodent model of nicotine self-administration, in which midbrain cholinergic and dopaminergic systems played a key role in motivating self-administration behavior (57).
A second finding was a nicotine dose-related decrease in normalized blood flow in the left hemisphere amygdala. Zubieta et al. (20) also found that nicotine reduced normalized blood flow in this region, but they detected a significant effect only in the right hemisphere amygdala. It is possible, given the role of the amygdala in affective responses, that effects of nicotine on this region might underlie some of the anxiolytic or antiaggressive effects of cigarette smoking. However, the subjective measures of the present studies did not reveal significant correlations with these effects.
A third result of the present studies related to the effect of nicotine on frontal regions. Both studies found an effect of nicotine in these regions, and in study 2, nicotine produced a decrease in right hemisphere relative to left hemisphere blood flow. This lateralized effect of nicotine is consistent with studies of electroencephalographic and electrodermal responses, which have suggested that nicotine tends to increase activation in the left hemisphere relative to that in the right hemisphere (10).
Apart from these overall findings from both studies, study 1 allowed us to assess the effects of intravenous nicotine administration and of inhaled nicotine (usual-brand of nicotine-containing cigarette). Intravenous and inhaled nicotine tended to have similar effects on normalized blood flow, although the effects of intravenous nicotine appeared to be not as robust as those of inhaled nicotine. A larger study group may be necessary to provide a quantitative comparison of the two routes of administration. Arterial sampling in study 1 also allowed us to assess the effects of nicotine on absolute blood flow. Only a subtle effect of intravenous nicotine was detected, in terms of a lateralized shift in blood flow (from left to right) related to nicotine dose, although global CBF tended to be lower overall in the nicotine conditions. Previous investigations have reported generalized decreases in regional cerebral metabolic rates for glucose after intravenous and intranasal nicotine administration (17, 18, 58). However, a study by Ghatan et al. (28) did not detect any effect on global blood flow of nicotine administered in a continuous intravenous infusion. However, absolute quantification of CBF was only performed with four subjects in that study.
Study 2 allowed us to investigate the effects of mecamylamine on nicotine response. Mecamylamine and nicotine had opposite effects on blood flow in the left hemisphere amygdala and the right hemisphere frontal regions. In the reticular system, mecamylamine attenuated the changes in normalized rCBF associated with nicotine.
The strengths of our experimental designs included individualizing nicotine dose to match that to which each subject was accustomed during ad lib smoking assessed at baseline. However, in concluding that there were nicotine dose-response relationships, it should also be acknowledged that a dose-response curve was not established for each subject. Thus, the relationship between nicotine dose and effect was based on observing responses across individuals who typically self-administer different doses when smoking a single cigarette. It is possible that dose-response relations observed for many of the dependent measures reflected individual differences in nicotine sensitivity or tolerance rather than a true dose-response effect. Future within-subjects dose-response analyses will be needed, for example, to determine to what extent a biphasic dose-response relation between nicotine and reticular system arousal is a common characteristic of smokers. Cumulative dosing over the course of a day is also likely to influence the effect on rCBF. The current studies administered doses typically obtained from a single cigarette over a 5–10-minute period, but in naturalistic settings, smokers take in a much higher nicotine dose over several hours.
One problem with the use of rCBF to assess the effects of nicotine is that nicotine may have direct vascular effects in a particular brain region, aside from the effects on neuronal activity. Mechanisms by which nicotine may affect vascular responses independently of regional brain metabolism include autonomic nervous system effects on blood vessel diameter and/or cardiac output, as well as actions mediated by intraparenchymal fibers from other brain regions, such as the locus ceruleus (59, 60). However, the present results indicate a specific anatomical pattern of changes elicited by nicotine that would be difficult to explain in terms of gross autonomic effects of the drug.
In summary, the results of these studies indicate potent nicotinic influences on rCBF. These effects were linked to functional neuroanatomical systems involved in attention, arousal, and reward. The influence of mecamylamine was generally opposite to that of nicotine. The relationship between changes in blood flow and dependent measures relevant to motivation, such as craving and self-reported reasons for smoking, suggests that further investigation of these regions is warranted to more fully understand tobacco addiction and to develop new potential avenues for treatment.
 |
Received Sept. 21, 2001; revision received Sept. 18, 2002; accepted Sept. 19, 2002. From the Veterans Affairs Medical Center, Durham, N.C.; the Departments of Psychiatry and Radiology, Duke University Medical Center; and the Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles. Address reprint requests to Dr. Rose, Nicotine Research Program, Duke University and VA Medical Centers, 2200 W. Main St., Suite B-150, Durham, NC 27705; [email protected] (e-mail). Supported by grant DA-10245 from the National Institute on Drug Abuse. The authors thank Sharon Hamblen, Mark P. Johnson, James E. Bates, and Tyler Buckner for technical assistance in conducting the study.
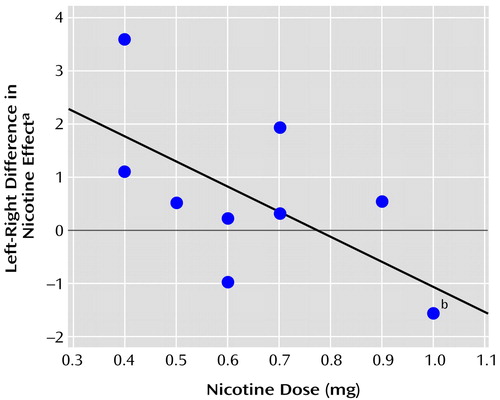
Figure 1. Relationship Between the Hemispheric Difference in the Effect of Nicotine on CBF and Nicotine Dose in Ten Healthy Cigarette Smokers
aEffect of nicotine indexed by the difference between CBF (cm3/minute per 100 g tissue) measured after intravenous infusion of nicotine and CBF measured after infusion of saline in subjects who smoked a denicotinized cigarette (y=3.69–4.85x).
bTwo subjects had a nicotine dose of 1.0 mg and a left-right difference in nicotine effect of –1.6.
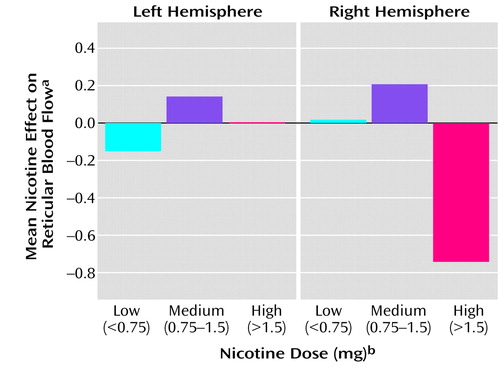
Figure 2. Mean Effect of Nicotine on Normalized Blood Flow in the Reticular System Factor as a Function of Nicotine Dose and Hemisphere in 32 Healthy Cigarette Smokers
aEffect of nicotine indexed as the difference between factor scores for CBF in the reticular system measured after subjects smoked a usual-brand (nicotine-containing) cigarette and for CBF after subjects smoked a denicotinized cigarette.
bN=14, N=15, and N=3 for the low-, medium-, and high-dose conditions, respectively.
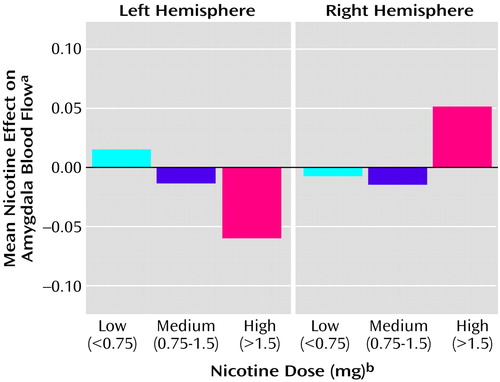
Figure 3. Mean Effect of Nicotine on Amygdala Normalized Blood Flow as a Function of Nicotine Dose and Hemisphere in 32 Healthy Cigarette Smokers
aEffect of nicotine indexed as the difference between normalized CBF in the amygdala measured after subjects smoked a usual-brand (nicotine-containing) cigarette and for normalized CBF after subjects smoked a denicotinized cigarette.
bN=14, N=15, and N=3 for the low-, medium-, and high-dose conditions, respectively.
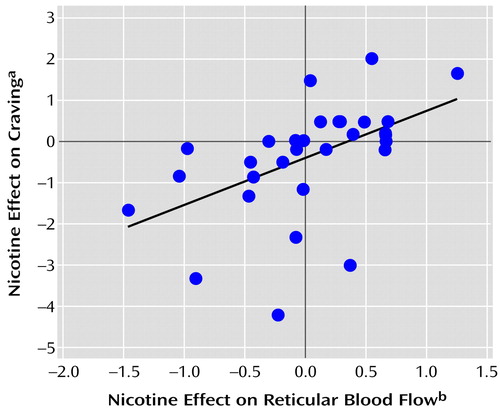
Figure 4. Relationship Between the Effect of Nicotine on Self-Reported Craving for Cigarettes and on Normalized Blood Flow in the Right Hemisphere Reticular System for 32 Healthy Cigarette Smokers
aEffect of nicotine on craving indexed as the difference between scores on a 7-point craving scale (based on a questionnaire about smoking withdrawal symptoms by Shiffman and Jarvik [39, 40]) measured after subjects smoked a usual-brand (nicotine-containing) cigarette and scores measured after subjects smoked a denicotinized cigarette.
bEffects of nicotine indexed as the difference between factor scores for CBF in the reticular system measured after subjects smoked a usual-brand (nicotine-containing) cigarette and for CBF measured after subjects smoked a denicotinized cigarette (y=–0.44+1.13x).
1. Jacobs DR, Adachi H, Mulder I, Kromhout D, Menotti A, Nissinen A, Blackburn H: Cigarette smoking and mortality risk. Arch Intern Med 1999; 159:733-740Crossref, Medline, Google Scholar
2. Peto R, Lopez AD, Boreham J, Thun M, Heath C, Doll R: Mortality from smoking worldwide. Br Med Bull 1996; 52:12-21Crossref, Medline, Google Scholar
3. US Department of Health and Human Services: The Health Consequences of Smoking: Nicotine Addiction. Rockville, Md, Office on Smoking and Health, 1988Google Scholar
4. Wonnacott S: Characterization of nicotine receptor sites in the brain, in Nicotine Psychopharmacology: Molecular, Cellular, and Behavioural Aspects. Edited by Wonnacott S, Russell MAH, Stolerman IP. New York, Oxford University Press, 1990, pp 226-277Google Scholar
5. Clarke PBS: The central pharmacology of nicotine: electrophysiological approaches. Ibid, pp 158-193Google Scholar
6. Alkondon M, Braga MF, Pereira EF, Maelicke A, Albuquerque EX: Alpha7 nicotinic acetylcholine receptors and modulation of GABAergic synaptic transmission in the hippocampus. Eur J Pharmacol 2000; 393:59-67Crossref, Medline, Google Scholar
7. McGehee DS, Heath MJS, Gelber S, Devay P, Role LW: Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 1995; 269:1692-1696Crossref, Medline, Google Scholar
8. Vidal C, Changeux J-P: Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neuroscience 1993; 56:23-32Crossref, Medline, Google Scholar
9. Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Oliney A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Walso MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W: Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 1997; 94:587-592Crossref, Medline, Google Scholar
10. Gilbert DG: Smoking: Individual Differences, Psychopathology, and Emotion. Washington, DC, Taylor & Francis, 1995Google Scholar
11. Levin ED, Conners CK, Sparrow E, Hinton SC, Meck WH, Rose JE, Erhardt D, March J: Nicotine effects on adults with attention deficit/hyperactivity disorder. Psychopharmacology (Berl) 1996; 123:55-63Crossref, Medline, Google Scholar
12. Pomerleau OF, Pomerleau CS: Research on stress and smoking: progress and problems. Br J Addict 1991; 86:599-604Crossref, Medline, Google Scholar
13. Stitzer ML, Gross J: Smoking relapse: the role of pharmacological and behavioral factors, in Nicotine Replacement: A Critical Evaluation. Edited by Pomerleau OF, Pomerleau CS. New York, Alan R Liss, 1988, pp 163-186Google Scholar
14. Grunwald F, Schrock H, Kuschinsky W: The effect of an acute nicotine infusion on the local cerebral glucose utilization of the awake rat. Brain Res 1987; 400:232-238Crossref, Medline, Google Scholar
15. London ED, Connolly R, Szikszay M, Wamsley J: Distribution of cerebral metabolic effects of nicotine in the rat. Eur J Pharmacol 1985; 110:391-392Crossref, Medline, Google Scholar
16. London ED, Connolly RJ, Szikszay M, Wamsley JK, Dam M: Effects of nicotine on local cerebral glucose utilization in the rat. J Neurosci 1988; 8:3920-3928Crossref, Medline, Google Scholar
17. Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF, Henningfield JE, London ED: Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology (in press)Google Scholar
18. Domino EF, Minoshima S, Guthrie SK, Ohl L, Ni L, Koeppe RA, Cross DJ, Zubieta JK: Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience 2000; 101:277-282Crossref, Medline, Google Scholar
19. Mathew RJ, Wilson WH: Acute pharmacological effects of tobacco smoking and nicotine on cerebral circulation, in Brain Imaging of Nicotine and Tobacco Smoking. Edited by Domino EF. Ann Arbor, Mich, NPP Books, 1995, pp 109-121Google Scholar
20. Zubieta J-K, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE, Koeppe RA, Domino EF: Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol Psychiatry 2001; 49:906-913Crossref, Medline, Google Scholar
21. Morioka C, Kondo H, Akashi K, Matsumura K, Ochi N, Makinaga G, Furukawa T: The continuous and simultaneous blood flow velocity measurement of four cerebral vessels and a peripheral vessel during cigarette smoking. Psychopharmacology (Berl) 1997; 131:220-229Crossref, Medline, Google Scholar
22. Skinhof E, Olosen J, Paulson OB: Influence of smoking and nicotine on cerebral blood flow and metabolic rate of oxygen in man. J Appl Physiol 1973; 35:820-822Crossref, Medline, Google Scholar
23. Wennmalm A: Effect of cigarette smoking on basal and carbon dioxide stimulated cerebral blood flow in man. Clin Physiol 1982; 2:529-535Crossref, Medline, Google Scholar
24. Nagata K, Shinohara T, Kanno I, Hatazawa J, Domino EF: Effects of tobacco cigarette smoking on cerebral blood flow in normal adults, in Brain Imaging of Nicotine and Tobacco Smoking. Edited by Domino EF. Ann Arbor, Mich, NPP Books, 1995, pp 95-107Google Scholar
25. Stein EA, Pankiewicz J, Harsch HH, Cho J-K, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS: Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 1998; 155:1009-1015Link, Google Scholar
26. Solti F, Peter A, Olah I, Iskum M, Hermann R, Refi Z: Effect of nicotine on cerebral blood flow and cerebral venous pressure. Cor Vasa 1963; 5:197-202Medline, Google Scholar
27. Cruikshank JM, Neil-Dweyer G, Dorrance DE, Hayes Y, Patel S: Acute effects of smoking on blood pressure and cerebral blood flow. J Hum Hypertens 1989; 3:443-449Medline, Google Scholar
28. Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J: Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology (Berl) 1998; 136:179-189Crossref, Medline, Google Scholar
29. Hasenfratz M, Baldinger B, Battig K: Nicotine or tar titration in cigarette smoking behavior? Psychopharmacology (Berl) 1993; 112:253-258Crossref, Medline, Google Scholar
30. Rose JE, Behm FM, Westman EC, Coleman RE: Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend 1999; 56:99-107Crossref, Medline, Google Scholar
31. Levin ED, Rose JE, Behm F: Controlling puff volume without disrupting smoking topography. Behav Res Methods Instrum Comput 1989; 21:383-386Crossref, Google Scholar
32. Benowitz NL, Porchet H, Jacob PI: Pharmacokinetics, metabolism, and pharmacodynamics of nicotine, in Nicotine Psychopharmacology: Molecular, Cellular, and Behavioural Aspects. Edited by Wonnacott S, Russell MAH, Stolerman IP. Oxford, UK, Oxford University Press, 1990, pp 112-157Google Scholar
33. Federal Trade Commission: Report of “Tar” and Nicotine Content of the Smoke of 145 Varieties of Cigarettes. Washington, DC, FTC, 1976Google Scholar
34. Gilbert DG, Jensen RA, Meliska CJ: A system for administering quantified doses of tobacco smoke to human subjects: plasma nicotine and filter pad validation. Pharmacol Biochem Behav 1989; 31:905-908Crossref, Google Scholar
35. DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE: Performance characteristics of a whole-body PET scanner. J Nucl Med 1994; 35:1398-1406Medline, Google Scholar
36. Meyer E: Simultaneous correction for tracer arrival delay and dispersion in CBF measurements by H215O autoradiographic method and dynamic PET. J Nucl Med 1989; 30:1069-1078Medline, Google Scholar
37. Pelizzari CA, Chen GTY, Spelbring DR, Weichselbaum RR, Chen CT: Accurate three-dimensional registration of CT, PET, and/or MR images of the brain. J Comput Assist Tomogr 1989; 13:20-26Crossref, Medline, Google Scholar
38. Turkington TG, Hoffman JM, Jaszczak RJ, MacFall JR, Harris CC, Kilts CD, Pelizzari CA, Coleman RE: Accuracy of surface fit registration for PET and MR brain images using full and incomplete brain surfaces. J Comput Assist Tomogr 1995; 19:117-124Crossref, Medline, Google Scholar
39. Shiffman SM, Jarvik ME: Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976; 50:35-39Crossref, Medline, Google Scholar
40. Westman EC, Levin ED, Rose JE: The nicotine patch in smoking cessation: a randomized trial with telephone counseling. Arch Intern Med 1993; 153:1917-1923Crossref, Medline, Google Scholar
41. Ikard FF, Green DE, Horn D: A scale to differentiate between types of smoking as related to the management of affect. Int J Addict 1969; 4:649-659Crossref, Google Scholar
42. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KL: The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86:1119-1127Crossref, Medline, Google Scholar
43. Maxwell SE, Delaney HD: Designing Experiments and Analyzing Data. Belmont, Calif, Wadsworth, 1990Google Scholar
44. Cohen J: Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
45. Stevens J: Applied Multivariate Statistics for the Social Sciences. Mahwah, NJ, Lawrence Erlbaum Associates, 1996Google Scholar
46. Davis M, Whalen PJ: The amygdala: vigilance and emotion. Mol Psychiatry 2001; 6:13-34Crossref, Medline, Google Scholar
47. Keppel G: Design and Analysis: A Researcher’s Handbook, 2nd ed. Englewood Cliffs, NJ, Prentice-Hall, 1982, p 699Google Scholar
48. Federal Trade Commission: Report of the Tar, Nicotine and Carbon Monoxide of the Smoke of 1,206 Varieties of Domestic Cigarettes for the Year 1994. Washington, DC, FTC, 1997Google Scholar
49. Jacob P, Wilson M, Benowitz NL: Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr 1981; 222:61-70Crossref, Medline, Google Scholar
50. Aubert I, Cecyre D, Gauthier S, Quirion R: Autoradiographic distribution of nicotinic receptor sites labelled with [3H]cytisine in the human brain, in Effects of Nicotine on Biological Systems II. Edited by Clarke PBS, Quik M, Adlkofer F, Thurau K. Basel, Switzerland, Birkhäuser, 1995, pp 363-369Google Scholar
51. Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S: Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther 1997; 282:7-13Medline, Google Scholar
52. Grady SR, Marks MJ, Collins AC: Desensitization of nicotine-stimulated [3H]-dopamine release from mouse striatal synaptosomes. J Neurochem 1994; 62:1390-1398Crossref, Medline, Google Scholar
53. Yin R, French ED: A comparison of the effects of nicotine on dopamine and non-dopamine neurons in the rat ventral tegmental area: an in vitro electrophysiological study. Brain Res Bull 2000; 51:507-514Crossref, Medline, Google Scholar
54. Mangan GL, Golding JF: The Psychopharmacology of Smoking. Cambridge, UK, Cambridge University Press, 1984Google Scholar
55. Ashton H, Marsh VR, Millman JE, Rawlins MD, Telford R, Thompson JW: Biphasic dose-related responses of the CNV (contingent negative variation) to IV nicotine in man. Br J Clin Pharmacol 1980; 10:579-589Crossref, Medline, Google Scholar
56. Dunn WL: Smoking as a possible inhibitor of arousal, in International Workshop on the Behavioral Effects of Nicotine. Edited by Bättig K. Basel, Switzerland, Karger, 1978, pp 18-25Google Scholar
57. Corrigall WA: Self-administered nicotine acts through the ventral tegmental area: implications for drug reinforcement mechanisms, in Effects of Nicotine on Biological Systems II. Edited by Clarke PBS, Quik M, Adlkofer F, Thurau K. Basel, Switzerland, Birkhäuser, 1995, pp 203-209Google Scholar
58. London ED, Morgan MJ: Positron emission tomographic studies on the acute effects of psychoactive drugs on brain metabolism and mood, in Imaging Drug Action in the Brain. Edited by London ED. Boca Raton, Fla, CRC Press, 1993, p 265Google Scholar
59. Hertz L: Autonomic control of neuronal-astrocytic interactions, regulating metabolic activities, and ion fluxes in the CNS. Brain Res Bull 1992; 29:303-313Crossref, Medline, Google Scholar
60. Lubsen N: Experimental studies on the cerebral circulation in the unanesthetized rabbit, III: the action of ergotamine tartrate and some vasodilator drugs. Archives Neerlandaises de Physiologie de l’Homme et des Animaux 1941; 25:361-365Google Scholar



