Functional Neuroanatomy of Grief: An fMRI Study
Abstract
OBJECTIVE: In this study the authors examined the functional neuroanatomy of grief, which to their knowledge has not been studied previously in functional neuroimaging research. METHOD: Grief was elicited in eight bereaved women through photographs of the deceased versus a stranger, combined with words specific to the death event versus neutral words. Use of both pictures and words resulted in a 2×2 factorial design. RESULTS: Three brain regions were independently activated by the picture and word factors: posterior cingulate cortex, medial/superior frontal gyrus, and cerebellum. The two factors also activated distinct regions: for the picture factor, they were the cuneus, superior lingual gyrus, insula, dorsal anterior cingulate cortex, inferior temporal gyrus, and fusiform gyrus; and for the word factor, they were the precuneus, precentral gyrus, midbrain, and vermis. The interaction of the two factors showed significant activation in the cerebellar vermis. CONCLUSIONS: Grief is mediated by a distributed neural network that subserves affect processing, mentalizing, episodic memory retrieval, processing of familiar faces, visual imagery, autonomic regulation, and modulation/coordination of these functions. This neural network may account for the unique, subjective quality of grief and provide new leads in understanding the health consequences of grief and the neurobiology of attachment.
Grief after the death of a loved one has received increased attention in psychiatry in recent years, with the realization that 40% of bereaved individuals suffer from major or minor depression (1) and are at increased risk for mortality from all causes (2). Bereaved individuals are at high risk for suicide (3), while treatment with antidepressants can lead to significant improvement in bereavement-related depression (4).
Despite this interest, to our knowledge grief has not been the subject of previous neuroimaging research. Findings from several related phenomena, however, provide direction regarding the possible neural correlates of grief. Recent functional imaging research has revealed that during the successful retrieval of autobiographical memories elicited by name-cued recall of family members and friends, the strongest and most consistent activation is in the caudal portion of the left posterior cingulate cortex (5). Perception of familiar faces and voices also is associated with increased neural activity in the posterior cingulate cortex, including the retrosplenial cortex (6). Transient sadness induced in a variety of ways has been associated with increased activity in the subgenual cingulate (Brodmann’s area 25) as well as the anterior insular cortex and prefrontal cortex (7, 8). In addition to other emotions evoked within a single positron emission tomography study, sadness induced by the free recall of a close relative’s or friend’s death was associated with activation of the bilateral insula, anterior cingulate cortex, orbitofrontal cortex, basal forebrain, caudate nucleus, lenticular nucleus, left thalamus, midline cerebellum, and anterior and dorsal pons (8).
We therefore predicted that in bereaved participants, the induction of transient grief would be associated with activity in the posterior cingulate cortex, anterior cingulate cortex, and prefrontal cortex. Given that grief is a complex cognitive, emotional, and physiological state, we anticipated that grief would be mediated by a distributed network of structures that would include, but not be restricted to, these areas.
In order to elicit grief, we used a 2×2 (person-by-word) factorial design, creating four conditions. Each of the four conditions consisted of 15 picture-word composites. By comparing a picture of the deceased with a picture of a stranger (person factor) and the personalized grief-related words with matched neutral words (word factor), we were able to elicit grief through two distinct methods in bereaved subjects.
Method
Participants
The participants were eight volunteers who had experienced the death of a first-degree relative in the past year. They were recruited through general advertisements in hospices, hospitals, mental health clinics, and university classes. All participants were female, right-handed, and native English speakers. The exclusion criteria included axis I psychiatric disorders (including current depression, based on condition A from DSM-IV) and medical conditions (including neurological disorders), which were evaluated with a structured clinical interview. The human subjects committee at the University of Arizona in Tucson approved the study. The participants provided written informed consent and were compensated for their participation.
Picture and Word Stimuli
Each participant provided one photograph of the deceased loved one. The deceased was the only or central figure in the photograph; any other persons were digitally removed from the image. The photograph was then matched for sex, age, and environment with one photograph of a stranger. The participant was also interviewed about the diagnosis, course of illness, death, and memorial service for the deceased. From this narrative, 15 personalized grief-related words were chosen that had an autobiographical connection to the death of the loved one (e.g., “collapse,” “funeral,” “loss”). These 15 grief-related words were then matched for part of speech, number of letters, and frequency of usage in the English language (9) with 15 neutral words (e.g., “announce,” “ceiling,” “list”).
The grief-eliciting procedure constituted a 2×2 (person-by-word) factorial design with four conditions. Each condition consisted of 15 picture-word composites. Each composite consisted of one picture paired with one word at the top of the screen. Condition 1 consisted of each of the 15 grief-related words, one per picture, presented simultaneously with the picture of the deceased. Condition 2 consisted of the 15 grief-related words paired one at a time with the picture of the stranger. Condition 3 consisted of the 15 neutral words paired one at a time with the picture of the deceased. Condition 4 consisted of the 15 neutral words paired with the picture of the stranger. Comparing conditions 1 and 3 (both with the picture of the deceased but differing in grief-related versus neutral words) with conditions 2 and 4 (both with the picture of the stranger but differing in the type of words) constituted the pictorial evocation of grief (“person factor”). Comparing conditions 1 and 2 (both with the grief-related words but differing in the picture of the deceased versus the stranger) with conditions 3 and 4 (both with neutral words but differing in the picture) constituted the verbal evocation of grief (“word factor”). Finally, the interaction of the person factor (deceased versus stranger) and the word factor (grief-related versus neutral) addressed the regulation of grief intensity in the context of different combinations of pictures and words.
Procedure
Imaging was performed 24 to 72 hours after the interview. The photographs were presented with Display Master DirectX (DMDX) (10) with a high-resolution stimulus presentation goggle system. Each picture was presented for an average of 9.0 seconds, with a range of 7.5 to 10.5 seconds. The screen was blank during the 0.5-second interval between pictures. The picture-word composites were presented in a random order with regard to the four conditions. The randomization procedure was constrained so that there were equal numbers of the four conditions every 20 pictures. The pictures were also counterbalanced so that half of the grief-related words were seen first with the deceased and half were seen first with the stranger. The average length of presentation of each picture was also random, within the range just noted. The 60 pictures were presented continuously for a total average duration of 10.5 minutes.
Skin conductance was monitored during picture presentation. We placed Ag-AgCl unpolarizable electrodes, designed for environments like magnetic resonance imaging, on the middle phalanx of the index and middle fingers on the left hand, and a skin conductance coupler provided a constant 0.5 V across electrodes. The psychophysiology setup was modified to work with functional MRI (fMRI). The electrodes were routed through a patch panel to amplifiers located outside the scanning room. The low-pass filter was set at 1.0 Hz, and the high-pass filter was set at 0.5 Hz to minimize electrical artifacts due to the changing magnetic gradients. Skin conductance responses were scored in an event-related analysis with AcqKnowledge software (AcqKnowledge version 3.5 for PC/Windows, BIOPAC Systems, Goleta, Calif.). The largest amplitude of the skin conductance response was measured from the window between 1 second after picture onset to 6 seconds after picture onset relative to the baseline for that stimulus. The baseline consisted of the skin conductance value 1 second after picture onset.
Immediately after the scanning, each participant viewed all 60 picture-word composites again for the same length of time and in the order that they were presented during imaging. The participants rated each composite on a scale of 1 to 10 for the peak intensity of grief experienced while viewing that composite during imaging. A rating of 1 represented no grief at all, and 10 represented the most intense grief that the subject had ever experienced in his or her life.
fMRI
Images were acquired on a 1.5-T GE Sigma whole-body Horizon system (GE Medical Systems, Milwaukee). Two localizer scans were followed by one 10.5-minute functional scan series. The functional scan series used a single-shot spiral sequence (11). Between 19 and 22 images were collected obliquely and aligned on the anterior commissure/posterior commissure plane, covering approximately the whole head (5-mm slice thickness, skip=1 mm, 3.44×3.44 in-plane resolution, TR=3000 msec, TE=40 msec, flip angle=90°). After completion of the functional scans, a T1-weighted set of images (256×256, TE=9 msec, TR=500 msec, field of view=22 cm, slice selection as for the functional data set, 4 minutes 20 seconds) was collected to provide anatomical reference images.
Analysis
The data were analyzed by using statistical parametric mapping (SPM99, Wellcome Department of Cognitive Neurology, University College London) implemented in MATLAB (Mathworks, Sherborn, Mass.). Automated algorithms were used to align each subject’s sequential MRI images, spatially normalize them into the stereotactic space of Talairach and Tournoux (12) by using the Montreal Neurological Institute standard brain (based on 305 brains), and smooth them (8 mm full width at half maximum). While the reported activation sites refer to the stereotactic coordinate system of Talairach and Tournoux (12), the localization was determined by using the atlas of Duvernoy (13).
Analysis was carried out by using the general linear model in an event-related analysis. Subject-specific low-frequency drift was removed by a high-pass filter, and global signal changes were removed by including a global covariate (14). Effects at each voxel were estimated, and regionally specific effects were compared by using linear contrasts. We investigated regional blood-oxygen-level-dependent (BOLD) changes independent of variations in whole brain measurements, and we generated separate normalized t score (i.e., z score) maps of BOLD increases during each of the four conditions. These t statistics constituted a statistical parametric map. Statistical inferences were based on a theory of random Gaussian fields (15). The contrasts for the individual subjects were aggregated for the group in a random effects analysis. BOLD changes were considered statistically significant if they equaled or exceeded a height threshold of z=3.09 (p<0.001, one-tailed, uncorrected).
The imaging data were first analyzed for main effects of the person factor—(condition 1 + condition 3) > (condition 2 + condition 4)—and the word factor—(condition 1 + condition 2) > (condition 3 + condition 4). The data were then analyzed for the interaction of the person and word factors.
Results
The mean duration of bereavement was 8.5 months (range=1–12). The participants were Caucasian (N=7) and Asian American (N=1). The deceased relatives were mother (N=4), father (N=3), and husband (N=1). The mean age was 41.9 years (range=19–58). All subjects reported that they were able to clearly see all stimuli.
The 60 skin conductance responses (obtained during scanning) and subjective ratings (obtained immediately after scanning) for each participant were transformed into z scores for this analysis, in order to determine the individual pattern of responses to the pictures across conditions, rather than the absolute magnitude of change. This rescaling allowed analyses of each individual’s responses according to her own average magnitude of response and degree of variability across trials.
Differences in physiological arousal among the conditions were indicated in the electrodermal responses, which are primarily an index of sympathetic nervous system activity. There was a significant difference among skin conductance responses (Figure 1), indicating that the grief-related stimuli elicited stronger electrodermal responses than the non-grief-related stimuli.
Across participants, the self-reported grief responses (on a 1–10 scale) to the picture-word composites were also significantly different for the four conditions. The mean scores were as follows: grief-related words plus photograph of deceased, 7.67 (range=4.87–9.93); grief-related words plus photograph of stranger, 4.23 (range=1.13–7.93); neutral words plus photograph of deceased, 3.52 (range=1.20–7.43); neutral words plus photograph of stranger, 1.75 (range=1.00–4.77). The z scores for the subjective grief responses were significantly different (Figure 2), indicating that the grief-related stimuli elicited stronger responses. The correlation of the skin conductance responses with the self-reported responses was significant (r=0.25, N=480, p<0.01).
The brain areas activated during the verbal evocation of grief (word factor) are listed in Table 1, and representative areas are displayed in Figure 3. The table demonstrates that verbally induced grief was associated predominantly with activity of the bilateral precuneus, the left medial frontal gyrus, the bilateral posterior cingulate gyrus, the right precentral gyrus, the midbrain (dorsal pons), and various cerebellar subregions.
Table 2 and Figure 4 show sites that were activated during the pictorial evocation of grief (person factor). Significant increases in neural activation centered on the left cuneus, the left posterior cingulate cortex/cuneus, the right superior lingual gyrus, the right insula, the right anterior cingulate cortex, the left superior frontal gyrus, the left inferior temporal gyrus, the left fusiform gyrus, and various cerebellar regions.
In the analysis of the interaction of the word factor and person factor, none of the predicted regions of interest (posterior cingulate cortex, anterior cingulate cortex, prefrontal cortex) showed significant activation. However, the cerebellar vermis (coordinates=6, –36, 2) showed significant activation (z score=3.69, cluster size=267; p<0.001, corrected).
In the single-subject analysis, the majority of the eight subjects showed a pattern of neural activation consistent with the predicted group results for verbal and pictorial evocation of grief. Five of the eight subjects showed increased neural activity in the posterior cingulate cortex/retrosplenial cortex (p≤0.05, uncorrected) associated with the word factor. In the cerebellum, seven subjects showed increased neural activity in the posterior lobe that was related to the person factor (p≤0.05, uncorrected). Seven subjects showed significant activation in the medial frontal gyrus associated with the person factor (p≤0.05, uncorrected). Five subjects revealed significant activation within the superior frontal gyrus in relation to the person factor (p≤0.05, uncorrected).
Discussion
In contrast to basic emotions such as sadness or fear, which may be elicited by using the same standard stimuli for all subjects, grief is a highly personal experience that is potentially difficult to elicit in the artificial neuroimaging environment. The picture-word composites used in this study to evoke grief were highly effective, as revealed by self-report and skin conductance data. Although the grief responses were genuine and intense, their duration was relatively brief, as indicated by the significant differences in skin conductance responses across the different conditions in this event-related paradigm. The combination of a general context (picture of the deceased or stranger) and a more specific memory elicitor (grief-related word or neutral word) was adapted from the procedure developed by Teasdale and colleagues (16), in which emotional responses were successfully elicited by congruent but not by incongruent picture-caption pairs.
Our findings revealed that while the combination of the picture of the deceased and the grief-related words evoked the most intense grief response, the picture of the deceased elicited a moderate grief response when paired with the neutral word. Similarly, the grief-related word elicited a moderate grief response even when paired with the picture of the stranger. As such, the person factor and the word factor provide different routes into the neurobiological state of grief. These two perspectives provide both similar and dissimilar information about the neural substrates of grief, analogous to a radiologic examination of a given anatomical structure from more than one viewing angle. Consequently, we discuss the findings for the word and person factors as complementary and overlapping. Collectively these factors indicate that grief is mediated by a distributed network of neural structures that subserve affect processing, mentalizing, retrieval of emotion-laden episodic memories, processing of familiar faces, visual imagery, automatic motor responses, autonomic regulation, and modulation/coordination of this combination of functions.
The caudal posterior cingulate/retrosplenial region was activated for both the person and word factors. This complex receives major hippocampal projections (17), has strong reciprocal connections with the parahippocampal and entorhinal cortices, and is intensely linked by reciprocal pathways to the dorsolateral prefrontal and anterior cingulate cortices. Thus, it may serve to connect the dorsolateral prefrontal cortex with the hippocampal formation. Activation of the caudal part of the posterior cingulate cortex is most strongly associated with the retrieval of autobiographical memories (5). Lesions of the left posterior cingulate cortex have also been associated with the loss of verbal episodic memory and retrograde amnesia for personal events. Indeed, low activity in this region is the most prominent functional imaging finding in the earliest stages of Alzheimer’s disease (18). In addition, the caudal posterior cingulate cortex is reciprocally connected to regions engaged in emotional processing, such as the anterior cingulate and orbitofrontal cortices. The posterior cingulate is consistently activated by emotionally salient stimuli and has been hypothesized to have a role in the interaction between emotion and memory (5). Maddock (19), in his review of the retrosplenial cortex, reported evidence that this structure participates in the encoding of the emotional significance of episodic memories, but he could not exclude its role in retrieval. The current study predominantly involved the latter and therefore supports its role in retrieval of emotion-laden episodic memories.
The precuneus was the area most robustly activated by the word factor. This region is part of the medial parietal cortex and is located superior and posterior to the retrosplenial cortex. It has widespread anatomical connections to the prefrontal cortex, the temporal and occipital cortices, and the thalamus (20). The precuneus is a principal area involved in the conscious recall of memory-related imagery (20) and may participate in the representation of polymodal imagery associated with successful memory retrieval (5). The activation of this area by the word factor, as opposed to the person factor, could be due to specific memories and visual images associated with actual events elicited by the grief-related words.
The person factor, but not the word factor, was associated with activation in a variety of extrastriate visual processing areas, including the superior lingual gyrus, the cuneus, and the fusiform gyrus. Activation of the inferior temporal gyrus also suggests enhanced activity in object-recognition areas in the context of grief, particularly areas involved in the recognition and processing of familiar faces. Emotional arousal induced by visual stimuli can itself modulate activity in visual processing areas (21, 22).
The person factor was also characterized by activity in the dorsal anterior cingulate cortex and the insula. The former is thought to play a superordinate role in executive control of attention (23). Activation of the dorsal anterior cingulate is consistent with our hypothesis that emotional arousal associated with grief engages attentional resources. Another paralimbic area, the anterior insula, is also well established as an area specialized for the processing of visceromotor information (24). Our procedure for eliciting grief was associated with significant skin conductance responses, supporting the interpretation that emotional arousal contributed to activation of these paralimbic regions.
Our finding of a significant activation in the left medial frontal gyrus (coordinates=–4, 62, 4) for the word factor corresponds to the regions associated with mentalizing, i.e., representing one’s own and others’ mental states (25, 26). This suggests that the participants reflected on their own state of mind, as well as that of the person in the picture, during the elicitation of grief.
Bowlby (27) viewed grief as a natural expression of what he called the “attachment behavioral system,” evoked to discourage prolonged separation of an individual from a primary attachment figure. Activation of the caudate nucleus was found during a functional neuroimaging study of romantic love (28). In the current study we also observed activation of the caudate nucleus (coordinates=6, 6, 8) that was marginally below the cutoff value of z=3.09 (p<0.001). Caudate activation may reflect activation of automatic motor programs, associated with the feeling of “being drawn toward” the person depicted. If so, the caudate nucleus may contribute to the attachment system hypothesized by Bowlby.
Growing evidence suggests that the cerebellum contributes to affective, autonomic, and cognitive functions as well as to motor control (8, 29). The cerebellar contribution to cognition is modulatory rather than generative. Damage to the cerebellar vermis leads to altered affectivity (30, 31). Our findings show that not only the cerebellar vermis, but also the posterior and anterior lobes of the cerebellum, may indeed play an essential role in the coordination of emotional and cognitive functioning in the context of grief, as they were activated in response to both the person and word factors.
A limitation of the study is the absence of a comparison group. Without a comparison group of nonbereaved women, it is possible that grief was confounded with familiarity or attachment. However, two fMRI studies have investigated brain activation in subjects while they were remembering familiar people (5) or viewing familiar faces and listening to familiar voices (6). Both demonstrated activation predominantly of the posterior cingulate/retrosplenial cortex, but in contrast to our results, no anterior cingulate or cerebellar activation was observed. The dorsal anterior cingulate cortex is greatly, but not exclusively, influenced by emotional processing and may correlate with phenomenal awareness of emotion (32), and the cerebellum may be the site where the intensity of emotional response is modulated (33). The activation of these two sites within our study may represent a fundamental functional correlate of the intense feeling of grief beyond mere familiarity with the person.
Because of the subjective nature of grief, which precludes studies in animals, and the lack of previous neuroimaging work addressing the neural correlates of grief, our exploratory hypotheses included multiple areas of the brain. The relatively small number of study participants (N=8) and the heterogeneity of their relationships to the deceased loved ones are also potentially confounding factors. The present study should therefore be considered preliminary and should be followed by a larger, more definitive study with appropriate controls.
Identifying the neural correlates of grief may contribute to the elucidation of the physiological effects of bereavement on physical health (34). For example, the “broken heart” phenomenon, the increased rate of sudden cardiac death within 6 months of conjugal bereavement, suggests that cardiovascular health is altered in bereavement (35, 36). Additional research is needed to determine whether grief and depression (36) exert adverse effects on cardiovascular health through similar mechanisms. Others have shown that traumatic grief symptoms predict a physical health event (e.g., cancer) months later (2). Psychiatry stands in a unique position to explore these effects of grief on mental and physical health and to improve the lives of those touched by this powerful human experience.
 |
 |
Received Nov. 15, 2002; revision received March 31, 2003; accepted April 8, 2003. From the Department of Psychiatry, University of Arizona, Tucson. Address reprint requests to Dr. Gündel, Institut und Poliklinik für Psychosomatische Medizin, Psychotherapie und Medizinische Psychologie, TU München, Klinikum rechts der Isar, Langerstrasse 3, D-81675 München, Germany; [email protected] (e-mail). Partially supported by a grant to the Arizona Alzheimer’s Research Center from the Arizona Department of Health Services and by a grant from American Death Educations and Counselors. The authors thank Ted Trouard for his expertise and assistance.
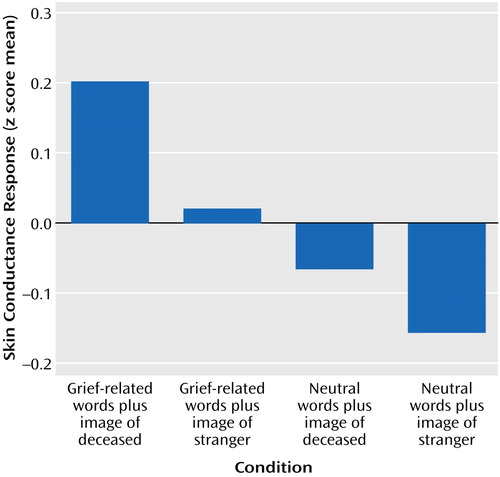
Figure 1. Skin Conductance Responses of Eight Bereaved Women Viewing Composites of Pictures and Words Related or Unrelated to the Deceased Relativea
aThe physiological responses were transformed into z scores to determine the pattern of responses and were then summed for each of the four conditions. The responses to the four conditions were significantly different (F=2.9, df=3, 476, p<0.03), indicating that the grief-related stimuli elicited stronger electrodermal responses than the non-grief-related stimuli.
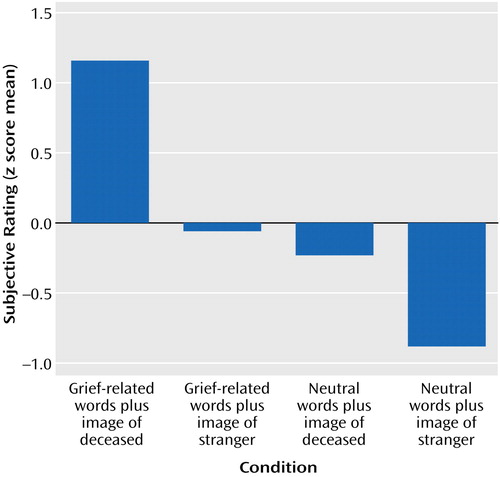
Figure 2. Subjective Grief Ratings by Eight Bereaved Women Viewing Pictures and Words Related or Unrelated to the Deceased Relativea
aThe ratings were transformed into z scores to determine the pattern of responses and were then summed for each of the four conditions. The responses to the four conditions were significantly different (F=197.7, df=3, 476, p<0.0001), indicating that the grief-related stimuli elicited stronger experiential responses than the non-grief-related stimuli.
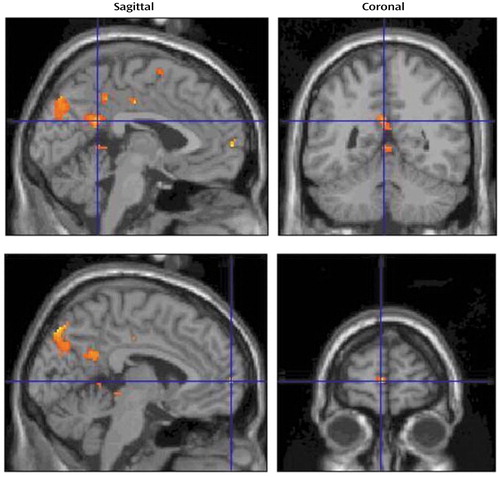
Figure 3. Brain Images Showing Activated Areas in Eight Bereaved Women Responding to Grief-Related Words (Word Factor) in Picture-Word Compositesa
aTop images: adjusted changes in blood-oxygen-level-dependent activity at the voxel of maximal activation in the left retrosplenial cortex (Talairach and Tournoux coordinates: x=–2, y=–50, z=24; z score=3.44; Brodmann’s area 30/31). Bottom images: changes in the medial frontal gyrus (Talairach and Tournoux coordinates: x=–4, y=62, z=4; z score=3.85; Brodmann’s area 9/10).
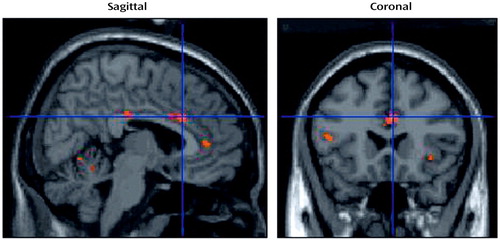
Figure 4. Brain Images Showing Activated Areas in Eight Bereaved Women Responding to a Photograph of the Deceased Relative (Person Factor) in Picture-Word Compositesa
aAdjusted changes in blood-oxygen-level-dependent activity at the voxel of maximal activation in the right dorsal anterior cingulate cortex (Talairach and Tournoux coordinates: x=6, y=22, z=28; z score=3.77; Brodmann’s area 24).
1. Zisook S, Paulus M, Shuchter SR, Judd LL: The many faces of depression following spousal bereavement. J Affect Disord 1997; 45:85–94Crossref, Medline, Google Scholar
2. Chen JH, Bierhals AJ, Prigerson HG, Kasl SV, Mazure CM, Jacobs S: Gender differences in the effects of bereavement-related psychological distress in health outcomes. Psychol Med 1999; 29:367–380Crossref, Medline, Google Scholar
3. Byrne GJ, Raphael B: Depressive symptoms and depressive episodes in recently widowed older men. Int J Geriatr Psychiatry 1999; 12:241–251Crossref, Google Scholar
4. Pasternak RE, Reynolds CF, Schlernitzauer M, Hoch CC, Buysse DJ, Houck PR, Perel JM: Acute open-trial nortriptyline therapy of bereavement-related depression in later life. J Clin Psychiatry 1991; 52:307–310Medline, Google Scholar
5. Maddock RJ, Garrett AS, Buonocore MH: Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neurosci 2001; 104:667–676Crossref, Medline, Google Scholar
6. Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR: The neural correlates of person familiarity: a functional magnetic resonance imaging study with clinical implications. Brain 2001; 124:804–815Crossref, Medline, Google Scholar
7. Phan KL, Wager T, Taylor SF, Liberzon I: Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002; 16:331–348Crossref, Medline, Google Scholar
8. Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD: Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci 2000; 3:1049–1056Crossref, Medline, Google Scholar
9. Francis WN, Kucera H: Frequency Analysis of English Usage: Lexicon and Grammar. Boston, Houghton Mifflin, 1982Google Scholar
10. Forster KI, Forster JC: DMDX: a Windows display program with millisecond accuracy. Behav Res Methods Instrum Comput 2003; 35:116–124Crossref, Medline, Google Scholar
11. Glover GH, Lee AT: Motion artifacts in fMRI: comparison of 2DFT with PR and spiral scan methods. Magn Reson Med 1995; 33:624–635Crossref, Medline, Google Scholar
12. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. Stuttgart, Germany, Georg Thieme, 1988Google Scholar
13. Duvernoy H: The Human Brain: Surface, Three-Dimensional Sectional Anatomy and MRI. New York, Springer-Verlag, 1991Google Scholar
14. Holmes AP, Josephs O, Buechel C, Friston KJ: Statistical modelling of low-frequency confounds in fMRI. Neuroimage 1998; 5:S480Google Scholar
15. Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
16. Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SCR, Checkley SA: Functional MRI study of the cognitive generation of affect. Am J Psychiatry 1999; 156:209–215Abstract, Google Scholar
17. Mesulam M-M: Behavioral neuroanatomy: large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations, in Principles of Behavioral and Cognitive Neurology. Edited by Mesulam M-M. London, Oxford University Press, 2000, pp 1–120Google Scholar
18. Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D: Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 1996; 334:752–758Crossref, Medline, Google Scholar
19. Maddock RJ: The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci 1999; 22:310–316Crossref, Medline, Google Scholar
20. Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RSJ, Dolan RJ: The mind’s eye—precuneus activation in memory-related imagery. Neuroimage 1995; 2:195–200Crossref, Medline, Google Scholar
21. Lane RD, Chua PML, Dolan RJ: Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia 1999; 37:989–997Crossref, Medline, Google Scholar
22. Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, Dolan RJ: A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 1998; 121:47–57Crossref, Medline, Google Scholar
23. Posner MI, Driver J: The neurobiology of selective attention. Curr Opin Neurobiol 1992; 2:165–169Crossref, Medline, Google Scholar
24. Augustine JR: Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev 1996; 22:229–244Crossref, Medline, Google Scholar
25. Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD: Reading the mind in cartoons and stories: an fMRI study of “theory of mind” in verbal and nonverbal tasks. Neuropsychologia 2000; 38:11–21Crossref, Medline, Google Scholar
26. Frith CD, Frith U: Interacting minds—a biological basis. Science 1999; 286:1692–1695Crossref, Medline, Google Scholar
27. Bowlby J: Attachment and Loss, vol III: Loss: Sadness and Depression. New York, Basic Books, 1980Google Scholar
28. Bartels A, Zeki S: The neural basis of romantic love. Neuroreport 2000; 11:3829–3834Crossref, Medline, Google Scholar
29. Schmahmann JD: From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp 1996; 4:174–198Crossref, Medline, Google Scholar
30. Schmahmann JD, Sherman JC: The cerebellar cognitive affective syndrome. Brain 1998; 121:561–579Crossref, Medline, Google Scholar
31. Dolan RJ: A cognitive affective role for the cerebellum. Brain 1998; 121:545–546Crossref, Medline, Google Scholar
32. Lane RD: Neural correlates of conscious emotional experience, in Cognitive Neuroscience of Emotion. Edited by Lane RD, Nadel L. London, Oxford University Press, 2000, pp 345–370Google Scholar
33. Damasio AR: Looking for Spinoza: Joy, Sorrow, and the Feeling Brain. Orlando, Fla, Harcourt, 2003Google Scholar
34. Hofer MA: Relationships as regulators: a psychobiologic perspective on bereavement. Psychosom Med 1984; 46:183–197Crossref, Medline, Google Scholar
35. Stroebe M: The broken heart phenomenon: an examination of the mortality of bereavement. J Community Appl Soc Psychol 1994; 4:47–61Crossref, Google Scholar
36. Eng PM, Rimm EB, Fitzmaurice G, Kawachi I: Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. Am J Epidemiol 2002; 155:700–709Crossref, Medline, Google Scholar



