Increase in Concentration of Waking Salivary Cortisol in Recovered Patients With Depression
Abstract
OBJECTIVE: Dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis with elevated plasma cortisol levels is characteristic of acute major depression. However, it is unclear whether HPA axis abnormalities are present in fully recovered patients. An increase in salivary cortisol levels after waking provides a simple, dynamic measure of HPA axis activity. The authors measured this increase in recovered depressed patients and in a healthy comparison group. METHOD: Salivary cortisol levels were measured upon waking and at 15-minute intervals for the next hour in 31 medication-free, recovered depressed patients and in 31 matched healthy comparison subjects. RESULTS: The increase in salivary cortisol levels that followed waking was significantly higher in the patients. CONCLUSIONS: Greater secretion of cortisol may be present in depressed subjects after clinical recovery and withdrawal of medication. This may put patients at risk of further episodes of depression as well as comorbid medical conditions, such as coronary heart disease.
Major depression is often accompanied by evidence of hypothalamic-pituitary-adrenal (HPA) axis dysfunction and elevated basal cortisol levels as well as abnormal cortisol responses to corticotropin-releasing hormone (CRH) (1). In general, abnormalities of the HPA axis are thought to normalize with clinical remission, perhaps in part because of antidepressant medications, which may facilitate HPA axis feedback by means of increased expression of glucocorticoid receptors (1, 2). Persistent abnormalities in HPA axis function in depressed patients are believed to indicate a high risk of depressive relapse (2). However, to our knowledge, there are no studies of cortisol secretion in unmedicated patients who meet strict criteria for full clinical recovery (3).
Measuring the increase in levels of plasma and salivary-free cortisol that follow awakening is a simple and reliable means of assessing the dynamic activity of HPA axis activity and may therefore offer advantages over isolated measures of basal salivary cortisol (4). In the present study, we assessed the waking increase in cortisol concentrations in a group of unmedicated recovered depressed patients to test the hypothesis that these subjects would have an exaggerated increase in salivary cortisol levels compared to healthy comparison subjects.
Method
In all, 66 subjects were recruited for the study. Two subjects in each group had to be excluded from the analysis because of inadequate sample volume or technical difficulties with the assay. Therefore, 31 patients (22 women and nine men; mean age=40.4 years, SD=15.2) and 31 comparison subjects (22 women and nine men; mean age=40.3 years, SD=14.0) were included in the analysis. The patients either had been treated previously in our department or were recruited by advertisement.
All of the patients had experienced at least two episodes of DSM-IV major depression in the past and had been euthymic and free of psychotropic drugs for at least 6 months (median=24, range=8–240). On the Structured Clinical Interview for DSM-IV disorders (SCID), 28 patients met criteria for recurrent major depression, while three met criteria for bipolar II disorder. The patients had a mean score on the 17-item Hamilton Depression Rating Scale of 2.0 (range=0–5) and a Beck Depression Inventory score of 4.9 (range=0–10). The comparison subjects were also screened on the SCID to exclude past or current axis I psychiatric illness. All subjects gave written informed consent for study participation, which was approved by the local psychiatric ethics committee.
Fasting saliva samples were collected in salivette tubes (Sarstedt, Leicester, U.K.), with the first sample taken immediately upon waking and continuing at 15-minute intervals for the next hour. The subjects followed a standard protocol for measurement of waking salivary cortisol and remained fasting (5). The time of the menstrual cycle in the female subjects was not controlled, but the premenstrual week was avoided; otherwise, the day of sampling was chosen by the subject. Salivary cortisol was measured with an in-house double antibody radioimmunoassay with intra- and interassay coefficients of variation of 3% and 10%, respectively. Statistical analysis employed analysis of variance (ANOVA) and unpaired t tests (two-tailed). Area under the curve was measured by the trapezoid method, with subtraction of baseline cortisol secretion. Analyses were carried out by means of Pearson’s product-moment correlations.
Results
There were no main or interactive effects of gender in any of the analyses. The mean time of awakening did not differ between the patients (7:26 a.m. ±16 minutes) and the comparison subjects (7:23 a.m. ±18 minutes). Baseline cortisol concentration at the time of awakening was similar in the comparison subjects and the recovered depressed subjects (mean=22.5 nmol/liter, SD=8.1, versus mean=20.0 nmol/liter, SD=8.4) (t=1.22, df=60, p=0.23). The ANOVA of the change from baseline showed a main effect of time (F=6.56, df=3, 180, p=0.002) and a main effect of group (F=6.73, df=1, 60, p<0.02). The group-by-time interaction was not significant (F=1.41, df=3, 60, p=0.25). The mean increase in salivary cortisol 30 minutes after waking in the recovered depressed subjects was double that of the comparison subjects (Figure 1). The area under the curve for cortisol secretion was also substantially greater in the recovered depressed subjects (mean=8.1 nmol × hour/liter, SD=7.9, versus mean=15.0 nmol × hour/liter, SD=12.1) (t=2.60, df=60, p<0.02). In the recovered depressed subjects, the area under the curve for cortisol level did not correlate with current scores on the Hamilton depression scale or the Beck Depression Inventory, the number of depressive episodes, or the number of periods of euthymia. In neither subject group was there a correlation between area under the curve for cortisol level and waking time or age (all p>0.10).
Discussion
Recovered depressed patients had significantly greater levels of waking salivary cortisol than age- and gender-matched comparison subjects. The waking increase in free cortisol is believed to reflect enhanced release of ACTH (6), which suggests that recovered depressed patients may have abnormalities in ACTH secretion or in the responsiveness of the adrenal gland to ACTH stimulation.
Our subjects were clinically recovered and medication free for long periods of time, so it seems unlikely that clinical symptom profiles or effects of antidepressant drugs can explain our findings. However, it is possible that mild residual symptoms not detected by our rating instruments could account for enhanced increases in waking cortisol levels. For example, in healthy volunteers, increased levels of perceived stress were associated with elevated waking salivary cortisol concentrations (5), and it is possible that recovered depressed patients may have a greater subjective experience of stress than healthy comparison subjects.
Our findings suggest that recovered depressed patients may demonstrate abnormalities in HPA axis function, leading to increased cortisol secretion at some time points during the day. It is possible that this abnormality may represent a trait marker of vulnerability to depression as is suggested by the changes in cortisol response to the combined CRH-dexamethasone test that is seen in subjects at high genetic risk of depression (7). Further studies will be needed to determine whether the exaggerated increase in waking salivary cortisol concentrations in recovered depressed subjects is associated with a risk of depressive relapse or a presentation of comorbid medical disorders, such as coronary heart disease, which have been previously linked to depression and hypercortisolemia (8).
Received Dec. 4, 2002; revision received May 27, 2003; accepted June 6, 2003. From the University Department of Psychiatry, Warneford Hospital. Address reprint requests to Dr. Cowen, the University Department of Psychiatry, Warneford Hospital, Oxford, OX3 7JX, U.K.: [email protected] (e-mail). Funded by the Medical Research Council (U.K.). The authors thank Alison Reed for technical assistance.
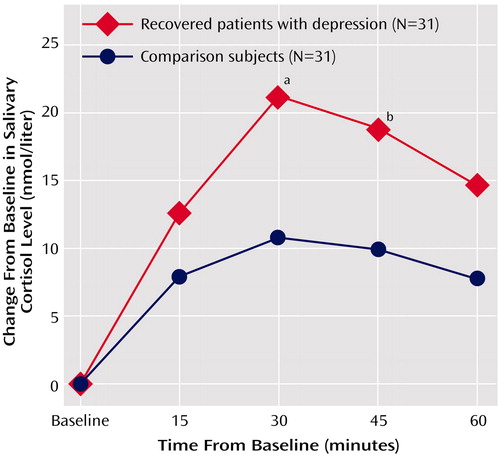
Figure 1. Mean Increase in Salivary Cortisol Levels After Waking in Recovered Patients With Depression and Comparison Subjects Matched by Age and Gender
aSignificant difference between groups (t=2.81, df=60, p=0.007).
bSignificant difference between groups (t=2.48, df=60, p<0.02).
1. Holsboer F, von Bardeleben U, Wiedemann K, Müller OA, Stalla GK: Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression: implications for pathophysiology of DST nonsuppression. Biol Psychiatry 1987; 22:228–234Crossref, Medline, Google Scholar
2. Holsboer F: Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord 2001; 62:77–91Crossref, Medline, Google Scholar
3. Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM: Conceptualization and rationale for consensus definition of terms in major depressive disorder: remission, recovery, relapse and recurrence. Arch Gen Psychiatry 1991; 48:851–855Crossref, Medline, Google Scholar
4. Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum AB, Von Auer K, Jobst S, Kaspers F, Kirschbaum C: Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 1997; 61:2539–2549Crossref, Medline, Google Scholar
5. Wüst S, Federenko I, Hellhammer DH, Kirschbaum C: Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology 2000; 25:707–720Crossref, Medline, Google Scholar
6. Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH, Kirschbaum C: The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci 1999; 64:1653–1660Crossref, Medline, Google Scholar
7. Modell S, Lauer CJ, Schreiber W, Huber J, Krieg J-C, Holsboer F: Hormonal response pattern in the combined DEX-CRH test is stable over time in subjects at high familial risk for affective disorders. Neuropsychopharmacology 1998; 18:253–262Crossref, Medline, Google Scholar
8. Gold PW, Drevets WC, Charney DS: New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biol Psychiatry 2002; 52:381–385Crossref, Medline, Google Scholar



