Brain Morphometric Abnormalities in Geriatric Depression: Long-Term Neurobiological Effects of Illness Duration
Abstract
OBJECTIVE: The authors’ goal was to compare regional brain volumes in depressed elderly subjects with those of nondepressed elderly subjects by using voxel-based morphometry. METHOD: They used statistical parametric mapping to analyze magnetic resonance imaging scans from 30 depressed patients 59 to 78 years old and 47 nondepressed comparison subjects 55 to 81 years old. RESULTS: Depressed patients had smaller right hippocampal volume than comparison subjects. The volume of the hippocampal-entorhinal cortex was inversely associated with the number of years since the first lifetime episode of depression. CONCLUSIONS: These data provide further evidence of structural brain abnormalities in geriatric depression, particularly in patients with a longer course of illness.
Neuroanatomical abnormalities among depressed elderly patients include volume reduction in the hippocampus (1), caudate nucleus (2), and prefrontal cortex (3). There is decreased glucose metabolism and cerebral blood flow in the prefrontal cortex (4), and neuropsychological studies have shown impaired executive function (5). Nevertheless, gaps of knowledge remain regarding the relationship between neuroanatomical abnormalities and the course of depression (see, for example, reference 6).
The purpose of this study was to compare regional gray and white matter volumes in depressed elderly subjects with those of nondepressed elderly subjects by using voxel-based morphometry (7). Voxel-based morphometry permits comprehensive, global assessment of brain structures without a priori identification of regions of interest. This approach is not biased toward any one brain region and permits identification of potential unsuspected brain structure abnormalities (8). Voxel-based morphometry may be more sensitive to within-structure neuroanatomical differences than the more traditional volumetric analyses that measure volumes of entire structures (9).
Method
The depressed subjects included in the study met DSM-IV criteria for current major depression and were enrolled in the Mental Health Intervention Research Center for Late-Life Mood Disorders (for details, see reference 10). Seventy-four of these patients had volumetric magnetic resonance imaging (MRI) scans at study entry. Thirty of these patients, who were equivalent in age and gender to our nondepressed comparison subjects, met the inclusion criterion of having no history of neurological disorder, untreated diabetes or hypertension, another axis I psychiatric disorder, substance abuse, or dementia. All of the patients included in the study had a Mini-Mental State Examination (MMSE) (11) score greater than 25 and a Mattis Dementia Rating Scale (12) score greater than 130. Volumetric MRI scans were also obtained from 47 nondepressed comparison subjects (for details of examinations and exclusion/inclusion criteria, see references 13–15). Written informed consent was obtained from all participating subjects after complete description of the study.
All subjects underwent a volumetric spoiled gradient recalled MRI sequence with a 1.5-T Signa scanner (GE Medical Systems, Madison, Wis.), yielding 120 contiguous coronal images. The normalized, segmented, and smoothed (8-mm isotropic Gaussian kernel) data were processed by using statistical parametric mapping (SPM 99) (see reference 8). The general linear model was applied to localize brain regional differences (for height threshold, p<0.001; extent threshold=30 contiguous voxels) and was corrected for multiple comparisons; age was entered as a covariate.
Results
There was no significant difference between the depressed and comparison subjects in terms of age (mean=69.3 years, SD=5.7, and mean=66.9 years, SD=7.3, respectively) (t=–1.51, df=75, p=0.14), gender (13 [43%] and 27 [57%] men, respectively) (χ2=1.44, N=77, p=0.23), race (29 [97%] and 42 [89%] Caucasian, respectively) (χ2=1.34, N=77, p=0.25), or MMSE score (mean=29.1, SD=1.0, and mean=29.4, SD=0.9, respectively) (t=0.87, df=75, p=0.38). The depressed patients had fewer years of education than the comparison subjects (mean=14.2 years, SD=3.1, and mean=15.7 years, SD=2.7, respectively) (t=2.33, df=75, p=0.02) and a mean of 1.8 lifetime episodes of major depression (SD=0.9, range=1–5). Depressed subjects had a mean Mattis Dementia Rating Scale (12) score of 139.4 (SD=2.7) and a mean 17-item Hamilton Depression Rating Scale (16) score of 15.6 (SD=7.1).
The depressed patients had significantly smaller gray matter volume of the right hippocampus and bilateral middle frontal gyrus than the comparison subjects (Figure 1). Depressed patients also had smaller white matter volume in the left anterior cingulate and right middle frontal gyrus.
Among the depressed patients only, on a voxel-by-voxel basis within SPM 99, we correlated the gray matter volume measure with the total number of years since the first lifetime episode of depression (using current age as covariate). The total elapsed time since the first episode ranged from 1 year (with onset at age 77) to 62 years (with onset at age 11). Two areas were identified in the right and left regions of the anterior inferior medial hippocampal gyrus, possibly including the entorhinal cortex (Figure 1). Partial correlation analyses (controlling for current age) found significant negative associations between the volumes of the right (r=–0.51, df=27, p=0.004) and left (r=–0.55, df=27, p=0.002) anterior hippocampal-entorhinal regions and years elapsed since depression onset. There was no association between anterior hippocampal-entorhinal volume and the number of depressive episodes, years of education, Hamilton depression scale score, Mattis Dementia Rating Scale score, or MMSE score.
Discussion
The depressed elderly subjects had significantly smaller hippocampal and prefrontal cortex volumes than nondepressed elderly subjects. The inverse correlation between the bilateral hippocampal-entorhinal volume and years since onset of depression is consistent with a study that found total lifetime duration (measured in days) to be significantly associated with hippocampal volume bilaterally (17). Our study extends these findings by identifying the anterior inferior mesial temporal lobe as the critical region of atrophy.
The apparent differential vulnerability of the anterior hippocampus and entorhinal cortex is provocative because the entorhinal region communicates with both the amygdala and the hippocampus. Disruption of these pathways impairs olfactory, visceral, and emotional processing (18), and pathological changes of the entorhinal region are associated with several neurodegenerative diseases. Our data suggest that the entorhinal region may play an important role in illness course and may have implications for the neurobiological treatment of the disease, including the use of cholinesterase inhibitors in conjunction with antidepressant pharmacotherapy.
Limitations of this study include potential inaccuracies during the segmentation procedure resulting in erroneous assignment of brain tissue (8), between-group differences in education, and limited generalizability because of a high degree of patient selection criteria.
Nevertheless, these data add to the growing body of data concerning the neuroanatomical abnormalities associated with depression in later life, particularly in patients with more elapsed time since their first episode. Our finding that the number of years since the first episode of depression was inversely associated with mesial temporal volume supports the model that hippocampal damage occurs in response to repeated exposure to elevated cortisol levels (17). Overall, these data suggest that the region of the hippocampus is exposed to “sustained” neurobiological toxins in patients with depression, resulting in reduced cell integrity.
Received Nov. 5, 2001; revision received March 19, 2002; accepted March 21, 2002. From the Neuropsychology Research Program, Functional Imaging Research Program, Mental Health Intervention Research Center for Late-Life Mood Disorders, and the Departments of Psychiatry, Radiology, Neurology, and Psychology, University of Pittsburgh Medical Center. Address reprint requests to Dr. Bell-McGinty, Neuropsychology Research Program, Suite 830, Oxford Bldg., 3501 Forbes Ave., Pittsburgh, PA 15213. Supported in part by NIMH grants MH-52247 (Mental Health Intervention Research Center for the Study of Late-Life Mood Disorders, Dr. Reynolds, Director), MH-43832, MH-37869, MH-01210, MH-59945, MH-01684 (Career Development Award, Dr. Butters), MH-19986 (Dr. Bell-McGinty), and MH-01077 (Research Scientist Development Award, Dr. Becker); by National Institute on Aging grant AG-05133 (University of Pittsburgh’s Alzheimer Disease Research Center, S.T. DeKosky, Director); and by National Heart, Lung, and Blood Institute grant HL-57529 (Cognitive and Cerebrovascular Consequences of Hypertension, J.R. Jennings, principal investigator). The authors thank M. Zmuda and S. Bensasi for their assistance with data management.
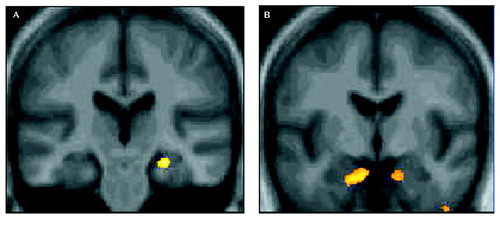
Figure 1. Regions of Smaller Gray Matter Volume in Depressed Subjects Than Nondepressed Subjects and Voxels Correlated With Depression Duration, With Controls for Chronological Agea
aThe images contain significant results (p<0.001) from statistical parametric mapping that controlled for current chronological age. Part A displays regions in which gray matter volume was smaller in depressed patients than in nondepressed comparison subjects. Results are projected in the coronal plane onto a mean image of the 47 elderly comparison subjects, showing the location of regional atrophy in the right hippocampus. Part B displays correlation analyses for depressed patients only, showing the negative association between volume and the number of years since the onset of the first episode of depression. Right and left regions of the anterior inferior medial hippocampal gyrus, possibly including the entorhinal cortex, are highlighted. (Images are displayed in neurologic convention.)
1. Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, MacFall JR, Krishnan KR: Hippocampal volume in geriatric depression. Biol Psychiatry 2000; 48:301-309Crossref, Medline, Google Scholar
2. Krishnan KR, Tupler LA, Ritchie JC, McDonald WM, Knight DL, Nemeroff CB, Carroll BJ: Apolipoprotein E-epsilon 4 frequency in geriatric depression. Biol Psychiatry 1996; 40:69-71Crossref, Medline, Google Scholar
3. Kumar A, Bilker W, Jin Z, Udupa J: Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology 2000; 22:264-274Crossref, Medline, Google Scholar
4. Nobler MS, Roose SP, Prohovnik I, Moeller JR, Louie J, Van Heertum RL, Sackeim HA: Regional cerebral blood flow in mood disorders, V: effects of antidepressant medication in late-life depression. Am J Geriatr Psychiatry 2000; 8:289-296Crossref, Medline, Google Scholar
5. Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J: Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry 2000; 57:285-290Crossref, Medline, Google Scholar
6. Mayberg HS: Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 1997; 9:471-481Crossref, Medline, Google Scholar
7. Ashburner J, Friston KJ: Voxel-based morphometry—the methods. Neurology 2000; 11:805-821Google Scholar
8. Gitelman DR, Ashburner J, Friston KJ, Tyler LK, Price CJ: Voxel-based morphometry of herpes simplex encephalitis. Neuroimage 2001; 13:623-631Crossref, Medline, Google Scholar
9. Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM: Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression: controlled magnetic resonance imaging study. Br J Psychiatry 1998; 172:527-532Crossref, Medline, Google Scholar
10. Mulsant BH, Pollock BG, Nebes R, Miller M, Sweet RA, Stack J, Houck PR, Bensasi S, Mazumdar S, Reynolds CF: A 12-week double-blind randomized comparison of nortriptyline and paroxetine in older depressed inpatients and outpatients. Am J Geriatr Psychiatry 2001; 9:406-414Crossref, Medline, Google Scholar
11. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
12. Mattis S: Dementia Rating Scale (DRS). Odessa, Fla, Psychological Assessment Resources, 1988Google Scholar
13. Lopez OL, Becker JT, Klunk W, Saxton J, Hamilton RL, Kaufer DI, Sweet RA, Meltzer CC, Wisniewski S, Kamboh MI, DeKosky ST: Research evaluation and diagnosis of possible Alzheimer’s disease over the last two decades, I. Neurology 2000; 55:1854-1862Crossref, Medline, Google Scholar
14. Mulsant BH, Pollock BG, Nebes RD, Miller MD, Little JT, Stack J, Houck PR, Bensasi S, Mazumdar S, Reynolds CF III: A double-blind randomized comparison of nortriptyline and paroxetine in the treatment of late-life depression:6-week outcome. J Clin Psychiatry 1999; 60(suppl 20):16-20Google Scholar
15. Jennings JR, Muldoon MF, Ryan CM, Mintun MA, Meltzer C, Townsend D, Sutton-Tyrell K, Shapiro AP, Manuck SB: Cerebral blood flow in hypertensives: an initial report of reduced and compensatory blood flow responses during performance of two cognitive tasks. Hypertension 1998; 31:1216-1222Crossref, Medline, Google Scholar
16. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
17. Sheline YI, Sanghavi M, Mintun MA, Gado MH: Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999; 19:5034-5054Crossref, Medline, Google Scholar
18. Mesulam M-M: Principles of Behavioral and Cognitive Neurology, 2nd ed. Oxford, UK, Oxford University Press, 2000Google Scholar



