Unaltered Dopamine Transporter Availability in Adult Attention Deficit Hyperactivity Disorder
Abstract
OBJECTIVE: The authors examined whether patients with attention deficit hyperactivity disorder (ADHD) have altered striatal dopamine transporter levels, which may explain psychostimulant effects in this disorder. METHOD: Single photon emission computed tomography and [123I]2β-carbomethoxy-3β-(4-iodophenyl)tropane ([123I]β-CIT) were used to assess dopamine transporter availability in nine adult patients with ADHD (eight of whom were stimulant naive) and nine age- and gender-matched healthy comparison subjects. RESULTS: Striatal [123I]β-CIT binding did not differ significantly between the ADHD and comparison subjects. CONCLUSIONS: The findings suggest that a hypothesized dysregulation of dopamine function in ADHD does not entail altered dopamine transporter levels.
Attention deficit hyperactivity disorder (ADHD) is a chronic neuropsychiatric condition characterized by inattention, hyperactivity, and impulsivity. Although the pathogenesis of ADHD remains unclear, alterations in central dopaminergic function have been hypothesized as a neurochemical basis for the disorder. Drugs that inhibit the dopamine transporter have widely demonstrated efficacy in the treatment of ADHD. Moreover, the dopamine transporter gene has been associated with ADHD in genetic linkage studies (1), and dopamine transporter knockout mice have been observed to exhibit motor overactivity (2). Therefore, an alteration in dopamine transporter concentrations might be suspected in ADHD. Two recent neuroimaging investigations using single photon emission computed tomography (SPECT) with [123I]altropane (3) and [99mTc]TRODAT-1 (4) have reported significant increases in dopamine transporter binding in adult ADHD patients.
In this study, we used the SPECT radioligand [123I]2β-carbomethoxy-3β-(4-iodophenyl)tropane ([123I]β-CIT) to examine the effects of adult ADHD on dopamine transporter and serotonin transporter availability. Pharmacological characterization of regional [123I]β-CIT uptake (5) has indicated that striatal activity is associated almost exclusively with the dopamine transporter, while binding in the brainstem and diencephalon is specific for the serotonin transporter. We tested the hypothesis that adults with ADHD have increased dopamine transporter availability and also undertook the first investigation, to our knowledge, of serotonin transporter availability in ADHD.
Method
The study group included nine patients with ADHD (six men, three women) who ranged in age from 25 to 56 years (mean=41 years, SD=11). All patients were recruited from the Yale Clinic for Attention Disorders in Adults where they were actively seeking evaluation and treatment. Patients were evaluated by a psychologist (D.M.Q.) and psychiatrist (C.H.vD.) to establish the diagnosis of ADHD according to the DSM-IV criteria and to exclude current neurological or psychiatric diseases, including substance abuse. The diagnoses were made by using contemporary information, including patients’ recollections of childhood behavior. Eight patients met the DSM-IV criteria for ADHD, combined type, and one for the predominantly inattentive type. Eight patients were stimulant naive; one had received methylphenidate intermittently between ages 9 and 11 but had received no stimulants in 14 years. The evaluation procedures for ADHD patients included a structured diagnostic interview with the Structured Clinical Interview for DSM-IV Axis I Disorders—Subject Edition (SCID-I/P) supplemented for childhood disorders by modules from the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (6). In addition, all patients were evaluated with the ADHD Rating Scale (7) modified for DSM-IV diagnostic criteria. Patients fulfilling inclusion/exclusion criteria returned for SPECT scanning within 3 weeks of the screening evaluation.
A comparison group was selected from a database of healthy subjects who had recently received [123I]β-CIT SPECT imaging. The comparison subjects were individually matched with the ADHD patients for age (±5 years, actual range=25–57 years, mean=41, SD=11), gender, hormone status (one female patient and one comparison subject—age 56 and 57 years, respectively—were postmenopausal and were not receiving hormone replacement therapy), and smoking status (all patients and comparison subjects were nonsmokers). Additional screening procedures for all patients and comparison subjects included evaluation with the SCID-I/P, physical and neurological examinations, ECG, routine blood and urine tests, and brain MRI. No subject was taking medication known to affect the central dopamine or serotonin systems. All subjects gave written informed consent for the study procedures. Subjects received 0.6 g of saturated potassium iodide oral solution (SSKI, Upsher-Smith Laboratories, Minneapolis) in the 24 hours before the scan.
All subjects received an injection of [123I]β-CIT (mean=5.9 mCi, SD=0.3), followed by SPECT scanning the next day (mean=23.0 hours after injection, SD=2.2). SPECT data acquisition and image analysis were performed as previously described (8). Briefly, simultaneous transmission and emission scans were acquired on a PRISM 3000 XP SPECT camera (Picker, Cleveland, Ohio). MRI scans of 3-mm contiguous transaxial slices were obtained with a 1.5-T Signa device (General Electric, Milwaukee). Image analysis was conducted by an operator (L.M.C.) who was unaware of the subject’s information. Nonuniform attenuation correction was performed by using the transmission scan, and MRI surface co-registration was performed in MEDx (Sensor Systems, Sterling, Va.) to guide the placement of standardized region-of-interest templates (for the striatum, diencephalon, brainstem, and cerebellum) on the corresponding SPECT slices.
Previous studies have demonstrated that [123I]β-CIT reaches equilibrium binding in the brain by 18–24 hours after injection (9), yielding the following simple unitless ratio of regional radioactivities in estimating the maximum number of dopamine transporter or serotonin transporter binding sites (i.e., Bmax):

where “ ” refers to the specific binding/nondisplaceable binding ratio, and “cpm” refers to counts per minute. Values of
” refers to the specific binding/nondisplaceable binding ratio, and “cpm” refers to counts per minute. Values of  for the striatum, diencephalon, and brainstem for the two diagnostic groups were compared with both two-sample and paired t tests as well as with analysis of covariance (ANCOVA), with age controlled. The relationship between V3′′ and behavioral measures within the ADHD group was examined with Pearson’s product-moment correlation.
for the striatum, diencephalon, and brainstem for the two diagnostic groups were compared with both two-sample and paired t tests as well as with analysis of covariance (ANCOVA), with age controlled. The relationship between V3′′ and behavioral measures within the ADHD group was examined with Pearson’s product-moment correlation.
Eight ADHD patients elected open-label treatment with methyphenidate after the SPECT scan. Methylphenidate was administered twice daily and titrated to a dose of 1.0 mg/kg per day or until limiting side effects occurred. The ADHD Rating Scale and the Clinical Global Impression of change (7-point Likert-type scale ranging from –3, very much worse, to 3,very much improved) were administered when the patients attained maximal dosages.
Results
Striatal [123I]β-CIT SPECT data are displayed in Figure 1. Mean values of striatal  did not differ between the healthy group (mean=6.27, SD=1.12) and the ADHD group (mean=6.25, SD=0.54) (t=0.04, df=16, p=0.97, two-sample t test; t=0.04, df=8, p=0.97, paired t test). Mean values of
did not differ between the healthy group (mean=6.27, SD=1.12) and the ADHD group (mean=6.25, SD=0.54) (t=0.04, df=16, p=0.97, two-sample t test; t=0.04, df=8, p=0.97, paired t test). Mean values of  did not differ between groups for the diencephalon (healthy subjects: mean=1.77, SD=0.33; ADHD subjects: mean=1.83, SD=0.27) (t=0.43, df=16, p=0.67, two-sample t test; t=0.47, df=16, p=0.65, paired t test) or the brainstem (healthy subjects: mean=1.13, SD=0.11; ADHD subjects: mean=1.14, SD=0.20) (t=0.11, df=16, p=0.92, two-sample t test; t=0.10, df=16, p=0.92, paired t test).
did not differ between groups for the diencephalon (healthy subjects: mean=1.77, SD=0.33; ADHD subjects: mean=1.83, SD=0.27) (t=0.43, df=16, p=0.67, two-sample t test; t=0.47, df=16, p=0.65, paired t test) or the brainstem (healthy subjects: mean=1.13, SD=0.11; ADHD subjects: mean=1.14, SD=0.20) (t=0.11, df=16, p=0.92, two-sample t test; t=0.10, df=16, p=0.92, paired t test).
When age was considered as a covariate, there was still no difference between groups in  for the striatum (F<0.01, df=1, 15, p=0.95), diencephalon (F=0.17, df=1, 15, p=0.69), or brainstem (F=0.01, df=1, 15, p=0.91). Although age has been significantly correlated in larger samples with
for the striatum (F<0.01, df=1, 15, p=0.95), diencephalon (F=0.17, df=1, 15, p=0.69), or brainstem (F=0.01, df=1, 15, p=0.91). Although age has been significantly correlated in larger samples with  for both the striatum (10) and the brainstem-diencephalon (11), age was not significant in the ANCOVA model for the striatum (t=–1.31, df=15, p=0.21), diencephalon (t=–1.03, df=15, p=0.32), or brainstem (t=0.19, df=15, p=0.85).
for both the striatum (10) and the brainstem-diencephalon (11), age was not significant in the ANCOVA model for the striatum (t=–1.31, df=15, p=0.21), diencephalon (t=–1.03, df=15, p=0.32), or brainstem (t=0.19, df=15, p=0.85).
For the eight patients who elected open-label methylphenidate treatment after SPECT imaging, a maximal daily dose of 0.60 mg/kg (SD=0.16, range=0.41–0.86) was achieved by a mean of 37 days (SD=22, range=11–78). During treatment, the mean ADHD Rating Scale score fell from 35.5 (SD=5.7, range=29–44) at baseline to 19.0 (SD=7.4, range=8–32) at maximal dose, with a mean reduction of 16.5 (SD=10.8, range=6–36). The mean Clinical Global Impression of change score was 1.9 (SD=0.8, range=1–3). One patient discontinued methylphenidate after 11 days because of side effects; another after 2 months because of noncompliance. Six patients continued to take methylphenidate or other stimulants at the time this report was written, 17–25 months later.
Within the ADHD patients, striatal  was uncorrelated with either baseline symptom severity as measured by the ADHD Rating Scale (mean=34.1, SD=6.8, range=23–44; r=0.11, N=9, p=0.77) or with symptom improvement (reduction in the ADHD Rating Scale score) during open-label methylphenidate treatment (r=0.17, N=8, p=0.68).
was uncorrelated with either baseline symptom severity as measured by the ADHD Rating Scale (mean=34.1, SD=6.8, range=23–44; r=0.11, N=9, p=0.77) or with symptom improvement (reduction in the ADHD Rating Scale score) during open-label methylphenidate treatment (r=0.17, N=8, p=0.68).
Discussion
The SPECT measures of [123I]β-CIT availability in adults with ADHD did not differ from those of matched comparison subjects. It is difficult to explain the striking discrepancy between our results and those of Dougherty et al. (3), who found a 70% higher age-corrected dopamine transporter density in six adult ADHD patients than in healthy comparison subjects. All but one of the patients in our study were stimulant naive, whereas Dougherty and colleagues subsequently disclosed that four of the six patients in their study had been previously treated with psychostimulants, although not within 1 month of study participation (12). Abstinence from stimulants might be associated with higher dopamine transporter levels, as has been observed in some neuroimaging studies of acute abstinence from cocaine (13). However, it is unlikely that this explanation could account for the 70% difference reported by Dougherty et al. (3). Moreover, stimulant effects cannot account for the difference between our results and those of Dresel et al. (4), who found that reportedly drug-naive adult ADHD patients (N=17) had a 17% higher level of dopamine transporter specific binding than healthy comparison subjects; the binding level for the patients decreased by 43% with methylphenidate treatment (4). Radioligand differences are also implausible sources of divergence. Although [123I]altropane and [99mTc]TRODAT-1, used in the studies by Dougherty et al. (3) and Dresel et al. (4), respectively, both possess greater selectivity for the dopamine transporter than for the serotonin transporter, [123I]β-CIT uptake in the striatum is associated almost exclusively with the dopamine transporter (5).
We cannot exclude the possibility that our study group was too small to detect a significant difference between diagnostic groups. However, on the basis of the mean value and standard deviation for striatal  in our pooled study group (mean=6.26, SD=0.86), our study had >99% power to detect a 70% difference between subject groups (the result reported by Dougherty et al. [3]), and approximately 80% power to detect even the 17% difference between groups reported by Dresel et al. (4) (alpha=0.05, two-tailed t test). Nonetheless, additional investigations are warranted with larger groups of stimulant-naive patients to clarify the discrepancy among these three preliminary studies.
in our pooled study group (mean=6.26, SD=0.86), our study had >99% power to detect a 70% difference between subject groups (the result reported by Dougherty et al. [3]), and approximately 80% power to detect even the 17% difference between groups reported by Dresel et al. (4) (alpha=0.05, two-tailed t test). Nonetheless, additional investigations are warranted with larger groups of stimulant-naive patients to clarify the discrepancy among these three preliminary studies.
This negative result does not rule out the possibility of an alteration of the brain’s dopamine system in ADHD. The absence of a difference in dopamine transporter availability does not preclude differences in dopamine transmission or dopamine receptors. Previous studies from our group have demonstrated differences in amphetamine-induced dopamine transmission (14) in patient populations (patients with schizophrenia) in which dopamine transporter availability did not differ (15). Additional studies should be conducted to examine stimulant-induced displacement of dopamine receptors in adults with ADHD.
Received May 18, 2001; revisions received July 13 and Sept. 17, 2001; accepted Sept. 25, 2001. From the Departments of Psychiatry and Diagnostic Radiology, Yale University School of Medicine; and West Haven Veterans Affairs Medical Center, West Haven, Conn. Address reprint requests to Dr. van Dyck, Cognitive Disorders Clinic, Department of Psychiatry, Yale University School of Medicine, One Church St., Suite 600, New Haven, CT 06510; christopher. [email protected] (e-mail).Supported by grants from Rose and Philip Hoffer, a Research Enhancement Award in Depression from the Department of Veterans Affairs, and NIMH grants MH-48243 and MH-58620.
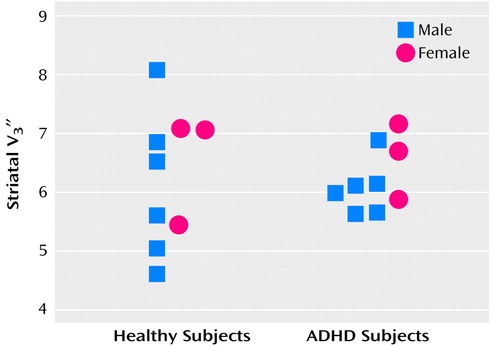
Figure 1. Striatal [123I]β-CIT Availability ( ) in Healthy Comparison Subjects (N=9) and Adults With Attention Deficit Hyperactivity Disorder (ADHD) (N=9), Individually Matched for Age and Gendera
) in Healthy Comparison Subjects (N=9) and Adults With Attention Deficit Hyperactivity Disorder (ADHD) (N=9), Individually Matched for Age and Gendera
aStriatal V3′′ did not differ between healthy and ADHD groups (t=0.04, df=16, p=0.97, two-sample t test; t=0.04, df=8, p=0.97, paired t test).
1. Cook EH, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL: Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet 1995; 56:993-998Medline, Google Scholar
2. Giros B, Jaber M, Jones SR, Wightman RM, Caron MG: Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996; 379:606-612Crossref, Medline, Google Scholar
3. Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ: Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 1999; 354:2132-2133Crossref, Medline, Google Scholar
4. Dresel S, Krause J, Krause K-H, LaFougere C, Brinkbäumer K, Kung HF, Hahn K, Tatsch K: Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med 2000; 27:1518-1524Crossref, Medline, Google Scholar
5. Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, Al-Tikriti MS, Sybirska EH, Zimmermann R, Wisniewski G, Neumeyer JL, Milius RA, Wang S, Smith EO, Roth RH, Charney DS, Hoffer PB, Innis RB: SPECT imaging of dopamine and serotonin transporters with [123I]β-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse 1993; 13:295-309Crossref, Medline, Google Scholar
6. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980-988Crossref, Medline, Google Scholar
7. DuPaul GJ, Power TJ, Anastopoulos AD, Reid R: ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, Guilford Press, 1998Google Scholar
8. Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB: Sex differences in [123I]β-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse 2001; 41:275-284Crossref, Medline, Google Scholar
9. Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea-Ponce Y, Zoghbi SS, Neumeyer JL, Charney DS, Hoffer PB, Innis RB: Graphical, kinetic, and equilibrium analyses of in vivo [ 123I]β-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab 1994; 14:982-994Crossref, Medline, Google Scholar
10. van Dyck CH, Seibyl JP, Malison RT, Wallace E, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Charney DS, Hoffer PB, Innis RB: Age-related decline in dopamine transporter binding in human striatum with [123I]β-CIT SPECT. J Nucl Med 1995; 36:1175-1181Medline, Google Scholar
11. van Dyck CH, Malison RT, Seibyl JP, Laruelle M, Klumpp H, Zoghbi SS, Baldwin RM, Innis RB: Age-related decline in central serotonin transporter availability with [123I]β-CIT SPECT. Neurobiol Aging 2000; 21:497-501Crossref, Medline, Google Scholar
12. Dougherty DD, Bonab AA, Spencer TJ, Madras BK, Fischman AJ: Dopamine-transporter density in patients with ADHD: Authors’ reply. Lancet 2000; 355:1461-1462Crossref, Medline, Google Scholar
13. Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB: Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I]β-CIT SPECT. Am J Psychiatry 1998; 155:832-834Abstract, Google Scholar
14. Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza C, Erdos J, McCance-Katz E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal J, Charney DS, Innis RB: SPECT imaging of amphetamine-induced dopamine activity in drug free schizophrenic subjects. Proc Natl Acad Sci USA 1996; 93:9235-9240Crossref, Medline, Google Scholar
15. Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, De Souza C, Krystal JH, Seibyl JP, Baldwin RM, Innis RB: Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [123I]β-CIT SPECT. Biol Psychiatry 2000; 47:371-379Crossref, Medline, Google Scholar



