Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex
Abstract
OBJECTIVE: Studies of the neurobiological processes underlying drug addiction primarily have focused on limbic subcortical structures. Here the authors evaluated the role of frontal cortical structures in drug addiction. METHOD: An integrated model of drug addiction that encompasses intoxication, bingeing, withdrawal, and craving is proposed. This model and findings from neuroimaging studies on the behavioral, cognitive, and emotional processes that are at the core of drug addiction were used to analyze the involvement of frontal structures in drug addiction. RESULTS: The orbitofrontal cortex and the anterior cingulate gyrus, which are regions neuroanatomically connected with limbic structures, are the frontal cortical areas most frequently implicated in drug addiction. They are activated in addicted subjects during intoxication, craving, and bingeing, and they are deactivated during withdrawal. These regions are also involved in higher-order cognitive and motivational functions, such as the ability to track, update, and modulate the salience of a reinforcer as a function of context and expectation and the ability to control and inhibit prepotent responses. CONCLUSIONS: These results imply that addiction connotes cortically regulated cognitive and emotional processes, which result in the overvaluing of drug reinforcers, the undervaluing of alternative reinforcers, and deficits in inhibitory control for drug responses. These changes in addiction, which the authors call I-RISA (impaired response inhibition and salience attribution), expand the traditional concepts of drug dependence that emphasize limbic-regulated responses to pleasure and reward.
Addiction is a complex disease process of the brain that results from recurring drug intoxication and is modulated by genetic, developmental, experiential, and environmental factors. The neurobiological changes that accompany drug addiction are not well understood. While until recently it was believed that addiction predominantly involved reward processes mediated by limbic circuits (see, for example, the reward deficiency syndrome [1]), results from recent neuroimaging studies have implicated additional brain areas, especially the frontal cortex. Here we summarize the findings from these neuroimaging studies and incorporate them with pertinent results from preclinical studies in suggesting a basis for an integrated model of drug addiction.
Most imaging studies have concentrated on the involvement of dopamine in the process of drug addiction because the ability of drugs of abuse to increase brain dopamine concentration in limbic brain regions is considered crucial for their reinforcing effects (2, 3). However, the increase in dopamine per se is insufficient to account for the process of addiction, since drugs of abuse increase dopamine in naive as well as in addicted subjects. In fact, in the case of cocaine addiction, the magnitude of drug-induced dopamine increases, and the intensity of self-reports of the drug’s reinforcing properties (the “high”), appears to be smaller in addicted than in naive subjects (4). This implies that dopamine involvement in drug addiction is likely to be mediated by means of functional and structural changes in the circuits that are modulated by dopamine, including the frontal cortex. In support of this suggestion are the findings of several recent structural/volumetric magnetic resonance imaging studies documenting morphological changes in the frontal lobe in various forms of drug addiction. For example, frontal lobe volume losses have been identified in cocaine-dependent subjects (5, 6), alcoholic subjects (7–9), and heroin-dependent subjects (5). The latter study noted negative correlations between normalized prefrontal volumes and years of either cocaine or heroin use, implying a cumulative effect of substance abuse on frontal volumes. Additional support is provided by studies done in rats. For example, it has been recently shown that self-administration of cocaine, but not food, results in morphological changes in dendrites and dendritic spines in the prefrontal cortex and nucleus accumbens (10). Dopamine activation, as seen during amphetamine administration, also suppresses the inhibition of the amygdala by the medial prefrontal cortex, possibly leading to a disinhibition of sensory-driven affective responses (11). A similar process may be occurring in human drug addiction, in which prefrontal top-down processes (see reference 12) are reduced, releasing behaviors that are normally kept under close monitoring and simulating stress-like reactions in which inhibitory control is suspended and stimulus-driven behavior is accentuated.
If the frontal cortex and its supervisory functions are indeed down-regulated in human drug addiction, the relevance of motivational, higher cognitive, and self-monitoring processes to this affliction cannot be overstated. Specifically, we propose that the behaviors and associated motivational states that are at the core of drug addiction are distinctly the processes of loss of self-directed/willed behaviors to automatic sensory-driven formulas and attribution of primary salience to the drug of abuse at the expense of other available rewarding stimuli. We hypothesize that these states are first evoked in the presence of the drug of abuse or cues conditioned to the drug but then become chronic action tendencies, contributing to relapse/bingeing (behavioral compulsion) and withdrawal/craving (mental compulsion, i.e., obsessiveness), respectively. We thus conceptualize drug addiction as a syndrome of impaired response inhibition and salience attribution and name it the “I-RISA” syndrome of drug addiction.
The I-RISA syndrome encompasses four clusters of behaviors that are interconnected in a positive feedback loop (Figure 1) and depend on the functioning of the prefrontal circuits vis-à-vis the subcortical reward pathway.
I-RISA Syndrome of Drug Addiction
Drug Intoxication
The process of short-term drug administration is one that has been traditionally associated with higher extracellular dopamine concentrations in limbic brain regions, in particular, the nucleus accumbens (13, 14). However, there is also evidence of increased concentration of dopamine in frontal regions (15).
Drug Craving
Craving is associated with the learned response that links the drug and its environment to a pleasurable or an intensely overpowering experience. The neuroanatomical substrates for consolidation of this memory are likely to involve the amygdala (16, 17) and the hippocampus (18), but activation of the thalamo-orbitofrontal circuit and the anterior cingulate might be a defining element in the actual experience of craving (19).
Compulsive Drug Administration
Compulsive drug self-administration in addicted individuals occurs even when the drug is no longer perceived of as pleasurable and in the presence of adverse physical reactions to the drug (20). This process of loss of control and drug bingeing is associated with dopaminergic, serotonergic, and glutamatergic circuits (21, 22) and probably involves the activation of the thalamo-orbitofrontal circuit and the anterior cingulate gyrus.
Drug Withdrawal
Recurrent drug administration and subsequent drug withdrawal result in disruption of behavioral circuits that culminate in dysphoria, anhedonia, and irritability (23), possibly contributing to relapse (24, 25). These changes are likely to involve disruption of frontal cortical circuits and neurotransmitters that include dopamine, serotonin, and corticotropin-releasing factor (26).
Involvement of the Frontal Cortex
Intoxication
Here we review the results from neuroimaging studies that have assessed the effects of drug administration on functional measures, such as glucose metabolism and cerebral blood flow (CBF). Few studies have measured regional brain activity during drug intoxication, and most of these studies have employed a single drug exposure. Such studies have shown lower glucose metabolism throughout the brain, including the frontal cortex, during cocaine, morphine, or alcohol intoxication (27–30). In contrast, marijuana intoxication is associated with higher levels of glucose metabolism in the prefrontal cortex, orbitofrontal cortex, and striatum in marijuana abusers but not in nonabusers (31). Similarly, faster metabolism in the prefrontal cortex, anterior cingulate, orbitofrontal cortex, and striatum has been reported in cocaine abusers after sequential administration of intravenous methylphenidate, which cocaine abusers report to be similar to intravenous cocaine (19). It should be noted that activation in the orbitofrontal cortex was only observed in the subjects in whom methylphenidate induced intense craving and in the prefrontal cortex in the subjects in whom it enhanced mood.
Studies measuring the effects of short-term drug administration on CBF have consistently reported higher levels of prefrontal CBF during intoxication with nicotine (32), marijuana (33), and alcohol (34–36). Moreover, the activation of the right prefrontal cortex during alcohol intoxication was associated with euphoria (35) and during marijuana intoxication with the subjective sense of intoxication (33). In contrast, cocaine lowered CBF throughout the brain, including the frontal cortex, an effect that could be attributed to cocaine’s vasoconstricting effects (37).
Mapping studies during drug intoxication with functional magnetic resonance imaging (fMRI) to measure the blood-oxygenation-level-dependent (BOLD) response have reported activation of the prefrontal cortex and anterior cingulate gyrus during cocaine intoxication, an effect that has been strongly correlated with drug reinforcement properties (38). Studies of nicotine administration have also shown activation in the frontal cortex and anterior cingulate gyrus coinciding in time with the subjective experiences of “rush” and “high” (39).
The discrepancies in activation patterns reported could reflect vasoactive and drug-specific effects or the difference in the temporal course of the processes measured (metabolism studies, 30 minutes; CBF-water studies, 60 seconds; BOLD studies, 3–5 seconds). Since the metabolism and CBF-water studies are limited by their poor time resolution, the BOLD method may be better suited for assessing the relationship between regional changes and drug-induced fast behavioral effects, such as “rush” and “high.” On the other hand, the BOLD method is limited by its sensitivity to vasoactive changes that may occur during drug administration.
To summarize, most of the studies show activation in the prefrontal cortex and anterior cingulate gyrus during drug intoxication when using either the CBF or BOLD methods. Also, prefrontal activation appears to be associated with the subjective perception of intoxication, the reinforcing effects of the drug, or enhanced mood. It is also intriguing that in the case of marijuana or methylphenidate, the activation of the frontal regions was predominantly observed in the abusers but not in the nonabusing subjects. This suggests that prefrontal regions and the anterior cingulate are involved in the intoxication process and that their response to drugs is in part related to previous drug experiences.
Craving and Bingeing
Acute drug administration is not necessary for the activation of the frontal cortex in individuals previously exposed to the drug of choice, in whom, because of prior exposure, craving alone is possibly sufficient to activate frontolimbic circuits. Thus, higher levels of brain activation (CBF, glucose metabolism, or BOLD) in frontolimbic areas, primarily in the prefrontal cortex and anterior cingulate, has been demonstrated in cocaine abusers exposed to videotapes depicting drug-related stimuli (40–44). Self-reports of craving significantly correlated with glucose metabolism changes in the dorsolateral prefrontal cortex in one study (42) and with the spatial extent of activation in the dorsolateral prefrontal cortex and anterior cingulate in another (43). In all five studies, the drug-related stimuli elicited craving only in the cocaine abusers and not in the comparison subjects, again pointing to the importance of prior drug experience.
The mechanism that underlies craving may entail recall of emotionally laden previous experiences. Indeed, craving correlated with activation of the amygdala in one study (42), and activation of the orbitofrontal cortex was observed when cocaine abusers recalled and described their own method of preparing cocaine but not when they described their family genealogical tree in a study from our laboratory (45) (Figure 2). In addition, craving may involve anticipation of a future drug reward. The role of the dorsolateral prefrontal cortex in the anticipation of immediate drug self-administration was previously suggested (42), although the dissociation of craving from anticipation and of both from the actual drug experience has not been supported. Nevertheless, the actual drug experience may be related with more circumscribed activations than the anticipation phase (42), in line with evidence for a similar dissociation of anticipation from an actual sensory (tactile) experience (46).
Another line of evidence supporting the role of the frontal cortex in craving derives from studies conducted shortly after the subjects’ last drug use. For example, we have demonstrated higher regional brain glucose metabolism, including in the orbitofrontal cortex and striatum, in cocaine abusers tested during early withdrawal (<1 week since last cocaine use) than in normal comparison subjects (47). These higher levels were proportional to the craving intensity, such that the higher the metabolism, the greater the drug craving. A central role for craving in orbitofrontal cortex activation has also been suggested by the results of a study from our laboratory in which methylphenidate increased orbitofrontal cortex and striatal metabolism only in the subjects in whom it enhanced craving (19).
The problem remains that cocaine craving is not a direct measure of compulsive cocaine use, and, in fact, its association with drug use and relapse continues to be challenged (48). Demonstrating the involvement of the orbitofrontal cortex in compulsive drug self-administration would require investigation of the abuser during actual use in which drug supply is unrestricted. Alternatively, a paradigm that simulates compulsive behavior (such as gambling when it is clearly no longer beneficial) might offer invaluable insight into the circuits underlying loss of control in addiction.
Withdrawal
Abnormalities in the human cortex associated with withdrawal from cocaine in regular cocaine abusers were documented as early as 1988 in our laboratory (49). We demonstrated that the relative CBF values for the prefrontal cortex and the left lateral frontal cortex were significantly lower in the cocaine users than in the normal comparison subjects. A follow up study in active cocaine abusers demonstrated differences in regional brain glucose metabolism between cocaine abusers tested within 1 week of last cocaine use and cocaine abusers tested 2–4 weeks after last cocaine use (47). Of interest, glucose metabolism was higher in the orbitofrontal cortex and in the striatum in the former group than in the normal comparison subjects. During more protracted withdrawal (1–6 weeks since last use), brain metabolism was found to be lower in cocaine abusers than in normal comparison subjects, an effect that was most accentuated in the frontal cortex (Figure 3) (50).
Studies of alcohol abusers have provided similar evidence. For example, glucose metabolism abnormalities (including in the frontal cortex) were documented in otherwise healthy alcoholic subjects with mean duration of alcohol withdrawal of 11 days (51). Persistent lower striatal metabolism has been shown in regional metabolism studies after more protracted alcohol withdrawal (52). In addition, alcoholic subjects have shown less sensitivity to the lower metabolism induced by lorazepam, a benzodiazepine that facilitates g-aminobutyric acid neurotransmission in the striatal-thalamo-orbitofrontal cortex circuit during early (1–4 weeks) detoxification (53) and in the orbitofrontal cortex during protracted (8–11 weeks) detoxification (54), suggesting long-lasting drug-related adjustments in these brain regions. Persistent abnormalities after alcohol detoxification were also documented for the anterior cingulate (54). Lower activity in the prefrontal cortex in alcoholic subjects during detoxification was also documented in other laboratories using slightly different study groups (Cloninger-type 2 alcoholics) and techniques (single photon emission computed tomography) (55).
Alcoholic subjects show less sensitivity in the striatal-thalamo-orbitofrontal cortex circuit to the serotonin agonist m-chlorophenylpiperazine, which provides evidence for the relevance of serotonin in these abnormalities (56). Studies from our laboratory also point to the relevance of dopamine in withdrawal. First, we documented that in cocaine abusers during early (up to 1 month since last cocaine use) and protracted (up to 4 months since last cocaine use) withdrawal, striatal dopamine response or receptor availability was significantly lower (4, 57, 58) than in normal comparison subjects. We also reported lower striatal dopamine D2 receptor binding in heroin (59) and methamphetamine (60) abusers and in alcoholic subjects (61) (Figure 4). Moreover, the lower levels of striatal D2 receptors were found to be associated with lower metabolism in the orbitofrontal cortex and anterior cingulate gyrus in cocaine addicted subjects (58) (Figure 5) and in the orbitofrontal cortex in methamphetamine abusers (62). Finally, higher metabolism in the anterior cingulate gyrus has been shown in response to methylphenidate, which increases dopamine by blocking the dopamine transporter (19), providing further support for the role of lower dopamine activation in frontal hypometabolism in drug addiction.
I-RISA Syndrome and Dopamine Circuits
For a review of the role of the mesolimbic and mesocortical dopamine system in inhibitory control and motivational processes in the rodent and primate brain and their putative impact on drug seeking, see Jentsch and Taylor (63). Here we will mention the mesolimbic and the mesocortical dopamine systems, which are classically associated with drug reinforcement and addiction. The mesolimbic dopamine circuit, which includes the nucleus accumbens, amygdala, and hippocampus, has been traditionally associated with the acute reinforcing effects of a drug and with the memory and conditioned responses that have been linked to craving. It is also likely to be involved in the emotional and motivational changes seen in drug abusers during withdrawal. The mesocortical dopamine circuit, which includes the prefrontal cortex, orbitofrontal cortex, and anterior cingulate, is likely to be involved in the conscious experience of drug intoxication, drug incentive salience, drug expectation/craving, and compulsive drug administration. Because these circuits operate in parallel and interact with one another, it is likely that a given behavior involves, to a greater or lesser extent, their joint participation. The nature of these interactions affects response to the drug. For example, the activation of memory circuits (the hippocampus and amygdala) in association with a drug-related context activates the orbitofrontal cortex and anterior cingulate in expectation of the reinforcer, which in turn activates the dopamine cells (64), leading to a further increase in the craving sensation and a possible decrease in inhibitory control. Note the circular nature of this interaction: the attribution of salience to a given stimulus, which is a function of the orbitofrontal cortex, depends on the relative value of a reinforcer compared to simultaneously available reinforcers (65), which requires knowledge of the strength of the stimulus as a reinforcer, a function of the hippocampus and amygdala. Consumption of the drug in turn will further activate cortical circuits (the orbitofrontal cortex and anterior cingulate) in proportion to the dopamine stimulation by favoring the target response and decreasing non-target-related background activity (66). The activation of these interacting circuits (Figure 6) may be indispensable for maintaining the compulsive drug administration observed during bingeing and to the vicious circle of drug addition (Figure 7).
A question remains why some people become addicted and others, under similar circumstances, do not. This question is one of the most challenging issues in drug abuse research. The neurobiological mechanisms underlying vulnerability to drug addiction are poorly understood and are likely to involve a balance between factors that confer vulnerability and those that protect against it. On the basis of imaging studies, one cannot rule out the possibility that the changes in frontal activity in drug-addicted subjects may have antedated their drug use and could have enabled the loss of control and compulsive drug intake in these subjects. But it is also possible that the changes in frontal activity are secondary to recurrent drug use and that other variables are responsible for the addiction vulnerability. We and others have hypothesized that the decrease in activity of D2 receptors may put individuals at risk for addictive behaviors as a means of temporarily compensating for the fewer D2-regulated reward circuits (67). Moreover, we have shown that overexpression of D2 receptors in the nucleus accumbens of rats previously trained to self-administer alcohol markedly reduces their alcohol intake (68). Since D2 receptor availability is positively associated with frontal activity in the human brain (reviewed in reference 69), this suggests that one of the mechanisms by which D2 receptors regulate drug self-administration, and possibly the potential for addiction, is by modulating frontal activity.
Neurocognitive Mechanisms
In the next several paragraphs, we summarize the functional neuroimaging studies conducted in healthy comparison subjects or non-drug-abusing populations that have recently implicated the striatal-thalamo-orbitofrontal cortex circuit in the I-RISA components. Specifically, we target perception of response-reinforcement relations and response inhibition, but we will also discuss the expectation of reward and depression. These four components can be viewed as intricately related to the four dimensions of our drug-addiction model (Figure 1), each potentially predisposing to drug addiction; thus
1. Drug intoxication is associated with the experience of its strong positive and negative reinforcement effects, an association that is strengthened through repeated self-administration and that possibly hinders the formation of similar associations; attribution of primary salience to the drug occurs at the expense of less powerful reinforcers.
2. We conceive impairment in response inhibition as underlying the experience of relapse and bingeing. When response-reinforcement regulation is down because of impaired salience attribution, response disinhibition, or impulsive responding to immediately salient, drug-related rewards is expected.
3. Expectation of the effects of the drug of abuse, whether it is the “high” or a lower negative state, is integral to drug craving.
4. Dysthymia is a core symptom of withdrawal, possibly reflecting adaptation responses to repeated dopamine enhancement by drugs of abuse in the reward circuits that render the latter less responsive to natural reinforcers (70–72). Behaviorally, this lower sensitivity in the reward circuits may represent a generalized impairment in the ability to derive pleasure from non-drug-related stimuli, leading to a state of anhedonia, which puts drug-addicted individuals at greater risk for seeking drug stimulation.
Response-Reinforcement Relations and Salience Attribution
Regarding perception of response-reinforcement relations, several recent fMRI studies have monitored brain hemodynamic changes during performance of game-playing tasks with monetary reinforcers (see reference 73 for review). Responses to monetary gains and losses or to winning and losing game points have been noted in the prefrontal cortex, orbitofrontal cortex, anterior cingulate, and thalamus. Activations of these areas in guessing, compared to reporting (the orbitofrontal cortex was exclusively activated in the guessing task in the more difficult condition, in which probability of being correct was down to 25% from 50%), point to a unique role of the correctness of a response and greater dependence on feedback under conditions of inherent uncertainty (74). Indeed, a recent fMRI study documented that unpredictability of a reward (water or juice) correlated with activity in the orbitofrontal cortex, thalamus, and nucleus accumbens (75). Positron emission tomography (PET) studies have similarly demonstrated striatal-thalamo-orbitofrontal cortex activations (including the anterior cingulate and dorsolateral prefrontal cortex) in association with gambling (76, 77), receiving a salient feedback (78), or receiving a monetary reward (79).
The processing of emotionally salient and behaviorally adaptive information may be at the core of advantageous assessment of response-reinforcement relations. Indeed, the role of the frontal cortex, and specifically the anterior cingulate, in emotional processing has been demonstrated in several PET studies (e.g., references 80–83). Consistent with these studies are the results of a fMRI study (84) in which the inferior frontal gyrus and dorsal anterior cingulate were involved in making a semantic, emotionally laden versus an orthographic decision in a verbal go/no-go task. Other fMRI studies have implicated the orbitofrontal cortex and anterior cingulate in the experience of pleasant sensations (85) and the orbitofrontal cortex in recognizing fearful, angry, and disgusted emotional facial expressions compared to neutral expressions (86). Of interest, in a PET study (87), angry but not sad faces specifically activated the orbitofrontal cortex, proportionally with the increasing intensity of the emotion, while the anterior cingulate cortex was coactivated by both expressions. Taken together, the results of these studies suggest an important integrative role for the orbitofrontal cortex and anterior cingulate in the analysis of the information that carries emotive, evaluative, and, in the long-term, survival significance for an individual, which comprise salience attribution, an integral part of our I-RISA syndrome of drug addiction.
Response Inhibition
The other component of the proposed I-RISA syndrome is the control of behavior, which is assumed to break down in periods of relapse and drug bingeing. Response inhibition has been relatively well studied in neuroimaging paradigms. For example, the orbitofrontal cortex, anterior cingulate cortex, and striatum were activated in a go/no-go task in two fMRI studies (88, 89). Better response inhibition was associated with greater volume of activation in the orbitofrontal cortex and a smaller magnitude of activation in the anterior cingulate cortex, possibly implicating the orbitofrontal cortex in the effort exerted when inhibiting a response and the anterior cingulate cortex in error detection (88). Further support for the role of the anterior cingulate in response inhibition, including response competition and selection, is provided by other fMRI (84, 90–92) and PET (e.g., reference 93) studies of the go/no-go paradigm. In addition, the role of the anterior cingulate in response inhibition has been established in studies of the suppression of prepotent response tendencies by using the Stroop effect (e.g., reference 94). We have recently provided more direct evidence for the role of the prefrontal circuit in response inhibition in drug addiction (95). We examined the association between Stroop interference and relative glucose metabolism in selected prefrontal brain regions in cocaine-addicted subjects, alcoholic subjects, and comparison subjects. The results revealed that for the cocaine-addicted subjects and alcoholic subjects, higher levels of orbitofrontal cortex metabolism at baseline was associated with lower conflict (higher Stroop interference score), while for the comparison subjects, higher orbitofrontal cortex metabolism was associated with higher conflict (lower Stroop interference score), suggesting a change in the role of the orbitofrontal cortex as a function of addiction.
Expectation
Supporting the role of the frontal cortex in expectation is an fMRI study that demonstrated distinct brain regions and different response characteristics in anticipation of pain versus the experience of pain, with the former activating more anterior regions (including the anterior medial frontal cortex) than the latter (46). Activation of the orbitofrontal cortex has also been associated with expectation in several PET studies, including expectation in tasks of visual attention (96) and in tasks involving a shock (97, 98).
Dysthymia
Finally, an association between depression and prefrontal abnormalities has been demonstrated in neuroimaging studies conducted in depressed patients, with suggested disruptions of frontostriatal (99) and corticolimbic (100) networks. Results of these studies revealed resting abnormalities in the dorsolateral, ventrolateral, and medial aspects of the prefrontal cortex and the anterior cingulate, blunted responses in the anterior cingulate and medial prefrontal cortex to behavioral and pharmacological challenges, and abnormalities localized to the orbitofrontal cortex (100, 101). Lower activity in the striatum of depressed patients in the resting state and in response to a reaction-time task and feedback have also been reported (102, 103).
Summary
The imaging studies reviewed here provide evidence for the involvement of the frontal cortex in the various aspects of drug addiction, including reinforcing responses to drugs during intoxication, activation during craving, and deactivation during withdrawal. The involvement of the frontal cortex throughout these cyclical stages of addiction is likely to play an important role in the cognitive behavioral and emotional changes that perpetuate drug self-administration and that are highlighted in the I-RISA syndrome of drug addition.
Future investigations should target the interplay between impaired salience attribution and response inhibition and their possible causal or predisposing effects toward developing drug addition.
Received Dec. 6, 2001; revision received April 18, 2002; accepted April 24, 2002. From Brookhaven National Laboratory. Address reprint requests to Dr. Goldstein, Brookhaven National Laboratory, Medical Research, Bldg. 490, 30 Bell Ave, Upton, NY 11973; [email protected] (e-mail). Supported in part by the U.S. Department of Energy (Office of Health and Environmental Research) under contract DE-ACO2-98CH10886, the National Institute of Drug Abuse (under grant DA-06891), and the Institute of Alcohol Abuse and Alcoholism (under grant AA-09481).
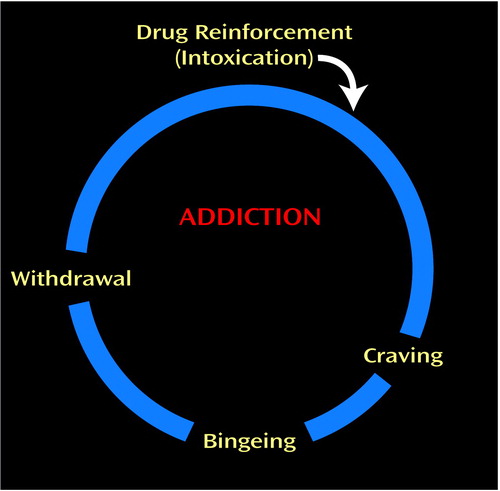
Figure 1. Behavioral Manifestations of the I-RISA (Impaired Response Inhibition and Salience Attribution) Syndrome of Drug Addiction
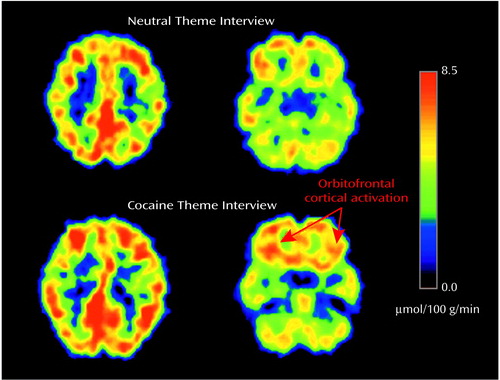
Figure 2. Orbitofrontal Cortical Activation in Active Cocaine Abusers During a Cocaine Theme Interview and a Neutral Theme Interview, as Measured by FDG PET
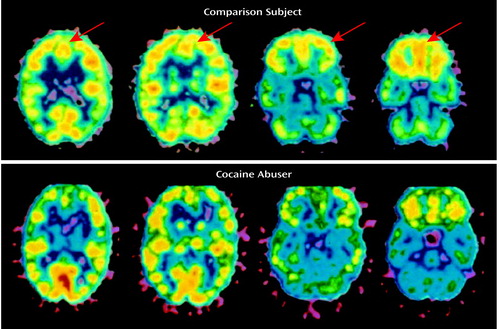
Figure 3. Lower Relative Glucose Metabolism in the Prefrontal Cortex and Anterior Cingulate Gyrus of a Cocaine Abuser Than in a Normal Comparison Subject
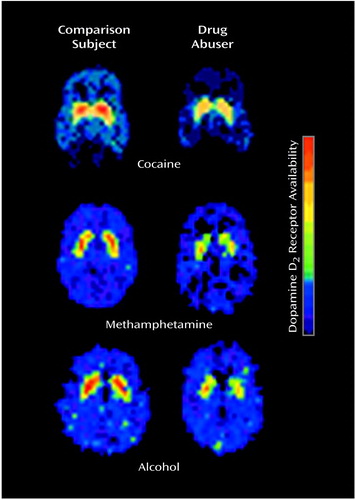
Figure 4. Lower Striatal Dopamine D2 Receptor Binding in Drug Users During Withdrawal From Cocaine, Methamphetamine, and Alcohol Than in Normal Comparison Subjects
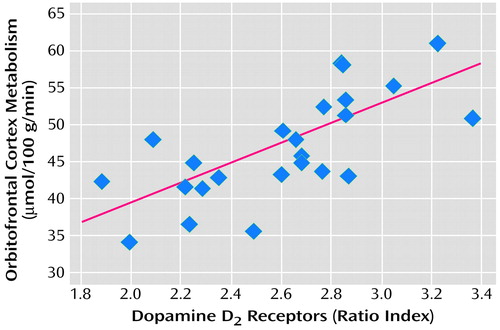
Figure 5. Relation of Striatal Dopamine D2 Receptor Availability and Orbitofrontal Cortical Metabolism in Cocaine Usersa,b
aAdapted from an earlier article (59). Reprinted from Synapse, ©1993 Wiley-Liss, Inc., with permission.
br=0.7, p<0.0001.
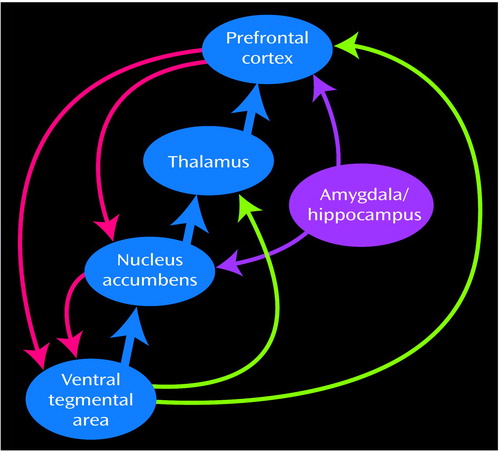
Figure 6. Interactions of the Mesocortical and Mesolimbic Circuits in Drug Addiction
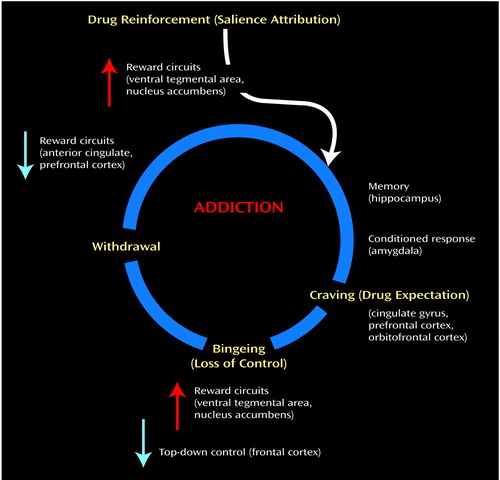
Figure 7. Integrative Model of Brain and Behavior: the I-RISA (Impaired Response Inhibition and Salience Attribution) Syndrome of Drug Addiction
1. Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE: Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 2000; 32(suppl i-iv):1-112Google Scholar
2. Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F: Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monogr 1994; 145:1-18Medline, Google Scholar
3. Di Chiara G: Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol 1999; 375:13-30Crossref, Medline, Google Scholar
4. Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N: Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 1997; 386:830-833Crossref, Medline, Google Scholar
5. Liu X, Matochik JA, Cadet JL, London ED: Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology 1998; 18:243-252Crossref, Medline, Google Scholar
6. Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR: Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 2002; 51:134-142Crossref, Medline, Google Scholar
7. Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS: Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res 1991; 15:418-427Crossref, Medline, Google Scholar
8. Jernigan TL, Schafer K, Butters N, Cermak LS: Magnetic resonance imaging of alcoholic Korsakoff patients. Neuropsychopharmacology 1991; 4:175-186Medline, Google Scholar
9. Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO: Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res 1997; 21:521-529Crossref, Medline, Google Scholar
10. Robinson TE, Gorny G, Mitton E, Kolb B: Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 2001; 39:257-266Crossref, Medline, Google Scholar
11. Rosenkranz JA, Grace AA: Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci 2001; 21:4090-4103Crossref, Medline, Google Scholar
12. Miller EK, Cohen JD: An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24:167-202Crossref, Medline, Google Scholar
13. Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ: Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 1987; 237:1219-1223Crossref, Medline, Google Scholar
14. Hurd YL, Ungerstedt U: Cocaine: an in vivo microdialysis evaluation of its acute action on dopamine transmission in rat striatum. Synapse 1989; 3:48-54Crossref, Medline, Google Scholar
15. Goeders NE, Smith JE: Reinforcing properties of cocaine in the medial prefrontal cortex: primary action on presynaptic dopaminergic terminals. Pharmacol Biochem Behav 1986; 25:191-199Crossref, Medline, Google Scholar
16. Brown EE, Fibiger HC: Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology (Berl) 1993; 113:123-130Crossref, Medline, Google Scholar
17. Meil WM, See RE: Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res 1997; 87:139-148Crossref, Medline, Google Scholar
18. Franklin TR, Druhan JP: Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci 2000; 12:2097-2106Crossref, Medline, Google Scholar
19. Volkow ND, Wang G-J, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding Y-S, Pappas N: Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry 1999; 156:19-26Link, Google Scholar
20. Fischman MW, Schuster CR, Javaid J, Hatano Y, Davis J: Acute tolerance development to the cardiovascular and subjective effects of cocaine. J Pharmacol Exp Ther 1985; 235:677-682Medline, Google Scholar
21. Loh EA, Roberts DC: Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology (Berl) 1990; 101:262-266Crossref, Medline, Google Scholar
22. Cornish JL, Duffy P, Kalivas PW: A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience 1999; 93:1359-1367Crossref, Medline, Google Scholar
23. Willner P, Muscat R, Papp M: Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 1992; 16:525-534Crossref, Medline, Google Scholar
24. Hodgins DC, el-Guebaly N, Armstrong S: Prospective and retrospective reports of mood states before relapse to substance use. J Consult Clin Psychol 1995; 63:400-407Crossref, Medline, Google Scholar
25. Johanson CE, Fischman MW: The pharmacology of cocaine related to its abuse. Pharmacol Rev 1989; 41:3-52Medline, Google Scholar
26. Koob GF, Le Moal M: Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001; 24:97-129Crossref, Medline, Google Scholar
27. Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, Dewey SL, Schlyer D, Burr G, Vitkun S, Hirschowitz J: Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res 1990; 35:39-48Crossref, Medline, Google Scholar
28. London ED, Broussolle EPM, Links JM, Wong DF, Cascella NG, Dannals RF, Sano M, Herning R, Snyder FR, Rippetoe LR, Toung TJK, Jaffe JH, Wagner HN Jr: Morphine-induced metabolic changes in human brain: studies with positron emission tomography and [fluorine 18]fluorodeoxyglucose. Arch Gen Psychiatry 1990; 47:73-81Crossref, Medline, Google Scholar
29. London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN Jr: Cocaine-induced reduction of glucose utilization in human brain: a study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry 1990; 47:567-574Crossref, Medline, Google Scholar
30. de Wit H, Metz J, Wagner N, Cooper M: Behavioral and subjective effects of ethanol: relationship to cerebral metabolism using PET. Alcohol Clin Exp Res 1990; 14:482-489Crossref, Medline, Google Scholar
31. Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L: Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res 1996; 67:29-38Crossref, Medline, Google Scholar
32. Nakamura H, Tanaka A, Nomoto Y, Ueno Y, Nakayama Y: Activation of fronto-limbic system in the human brain by cigarette smoking: evaluated by a CBF measurement. Keio J Med 2000; 49(suppl 1):A122-A124Google Scholar
33. Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE: Regional cerebral blood flow after marijuana smoking. J Cereb Blood Flow Metab 1992; 12:750-758Crossref, Medline, Google Scholar
34. Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S: Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res 1988; 24:201-209Crossref, Medline, Google Scholar
35. Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M, Hallikainen T: Acute ethanol-induced changes in cerebral blood flow. Am J Psychiatry 1994; 151:1505-1508Link, Google Scholar
36. Ingvar M, Ghatan PH, Wirsen-Meurling A, Risberg J, Von Heijne G, Stone-Elander S, Ingvar DH: Alcohol activates the cerebral reward system in man. J Stud Alcohol 1998; 59:258-269Crossref, Medline, Google Scholar
37. Wallace EA, Wisniewski G, Zubal G, vanDyck CH, Pfau SE, Smith EO, Rosen MI, Sullivan MC, Woods SW, Kosten TR: Acute cocaine effects on absolute cerebral blood flow. Psychopharmacology (Berl) 1996; 128:17-20Crossref, Medline, Google Scholar
38. Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE: Acute effects of cocaine on human brain activity and emotion. Neuron 1997; 19:591-611Crossref, Medline, Google Scholar
39. Stein EA, Pankiewicz J, Harsch HH, Cho J-K, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS: Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 1998; 155:1009-1015Link, Google Scholar
40. Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP: Limbic activation during cue-induced cocaine craving. Am J Psychiatry 1999; 156:11-18Link, Google Scholar
41. Garavan H, Pankiewicz J, Bloom A, Cho J-K, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA: Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 2000; 157:1789-1798Link, Google Scholar
42. Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A: Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 1996; 93:12040-12045Crossref, Medline, Google Scholar
43. Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF: Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 1998; 155:124-126Link, Google Scholar
44. Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC: Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 2001; 158:86-95Link, Google Scholar
45. Wang G-J, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C: Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 1999; 64:775-784Crossref, Medline, Google Scholar
46. Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN: Dissociating pain from its anticipation in the human brain. Science 1999; 284:1979-1981Crossref, Medline, Google Scholar
47. Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A: Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry 1991; 148:621-626Link, Google Scholar
48. Tiffany ST: A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev 1990; 97:147-168Crossref, Medline, Google Scholar
49. Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K: Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry 1988; 152:641-648Crossref, Medline, Google Scholar
50. Volkow ND, Hitzemann R, Wang G-J, Fowler JS, Wolf AP, Dewey SL, Handlesman L: Long-term frontal brain metabolic changes in cocaine abusers. Synapse 1992; 11:184-190Crossref, Medline, Google Scholar
51. Volkow ND, Hitzemann R, Wang G-J, Fowler JS, Burr G, Pascani K, Dewey SL, Wolf AP: Decreased brain metabolism in neurologically intact healthy alcoholics. Am J Psychiatry 1992; 149:1016-1022Link, Google Scholar
52. Volkow ND, Wang G-J, Hitzemann R, Fowler JS, Overall JE, Burr G, Wolf AP: Recovery of brain glucose metabolism in detoxified alcoholics. Am J Psychiatry 1994; 151:178-183Link, Google Scholar
53. Volkow ND, Wang G-J, Hitzemann R, Fowler JS, Wolf AP, Pappas N, Biegon A, Dewey SL: Decreased cerebral response to inhibitory neurotransmission in alcoholics. Am J Psychiatry 1993; 150:417-422Link, Google Scholar
54. Volkow ND, Wang G-J, Overall JE, Hitzemann R, Fowler JS, Pappas N, Frecska E, Piscani K: Regional brain metabolic response to lorazepam in alcoholics during early and late alcohol detoxification. Alcohol Clin Exp Res 1997; 21:1278-1284Crossref, Medline, Google Scholar
55. Catafau AM, Etcheberrigaray A, Perez de los Cobos J, Estorch M, Guardia J, Flotats A, Berna L, Mari C, Casas M, Carrio I: Regional cerebral blood flow changes in chronic alcoholic patients induced by naltrexone challenge during detoxification. J Nucl Med 1999; 40:19-24Medline, Google Scholar
56. Hommer D, Andreasen P, Rio D, Williams W, Ruttimann U, Momenan R, Zametkin A, Rawlings R, Linnoila M: Effects of m-chlorophenylpiperazine on regional brain glucose utilization: a positron emission tomographic comparison of alcoholic and control subjects. J Neurosci 1997; 17:2796-2806Crossref, Medline, Google Scholar
57. Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue C-Y, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, Hitzemann R, Henn F: Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 1990; 147:719-724Link, Google Scholar
58. Volkow ND, Fowler JS, Wang G-J, Hitzemann R, Logan J, Schlyer D, Dewey S, Wolf AP: Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 1993; 14:169-177Crossref, Medline, Google Scholar
59. Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K: Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology 1997; 16:174-182Crossref, Medline, Google Scholar
60. Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y-S, Logan J, Wong C, Miller EN: Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 2001; 158:377-382Link, Google Scholar
61. Volkow ND, Wang G-J, Fowler JS, Logan J, Hitzemann R, Ding Y-S, Pappas N, Shea C, Piscani K: Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 1996; 20:1594-1598Crossref, Medline, Google Scholar
62. Volkow ND, Chang L, Wang G-J, Fowler JS, Ding Y-S, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N: Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 2001; 158:2015-2021Link, Google Scholar
63. Jentsch JD, Taylor JR: Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999; 146:373-390Crossref, Medline, Google Scholar
64. Karreman M, Moghaddam B: The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem 1996; 66:589-598Crossref, Medline, Google Scholar
65. Schultz W, Tremblay L, Hollerman JR: Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex 2000; 10:272-284Crossref, Medline, Google Scholar
66. Kiyatkin EA, Rebec GV: Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol 1996; 75:142-153Crossref, Medline, Google Scholar
67. Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding Y-S, Pappas N: Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry 1999; 156:1440-1443Link, Google Scholar
68. Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R: Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem 2001; 78:1094-1103Crossref, Medline, Google Scholar
69. Volkow ND, Fowler JS: Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000; 10:318-325Crossref, Medline, Google Scholar
70. Cassens G, Actor C, Kling M, Schildkraut JJ: Amphetamine withdrawal: effects on threshold of intracranial reinforcement. Psychopharmacology (Berl) 1981; 73:318-322Crossref, Medline, Google Scholar
71. Barr AM, Phillips AG: Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999; 141:99-106Crossref, Medline, Google Scholar
72. Barr AM, Fiorino DF, Phillips AG: Effects of withdrawal from an escalating dose schedule of d-amphetamine on sexual behavior in the male rat. Pharmacol Biochem Behav 1999; 64:597-604Crossref, Medline, Google Scholar
73. Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P: Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 2001; 30:619-639Crossref, Medline, Google Scholar
74. Elliott R, Rees G, Dolan RJ: Ventromedial prefrontal cortex mediates guessing. Neuropsychologia 1999; 37:403-411Crossref, Medline, Google Scholar
75. Berns GS, McClure SM, Pagnoni G, Montague PR: Predictability modulates human brain response to reward. J Neurosci 2001; 21:2793-2798Crossref, Medline, Google Scholar
76. Grant SJ, Bonson KR, Contoreggi CC, London ED: Activation of the ventromedial prefrontal cortex correlates with gambling task performance: a FDG-PET study. Abstracts of the Society for Neuroscience 1999; 25(part 2):1551Google Scholar
77. Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW: Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 1999; 19:9029-9038Crossref, Medline, Google Scholar
78. Elliott R, Frith CD, Dolan RJ: Differential neural response to positive and negative feedback in planning and guessing tasks. Neuropsychologia 1997; 35:1395-1404Crossref, Medline, Google Scholar
79. Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL: Activation of the human brain by monetary reward. Neuroreport 1997; 8:1225-1228Crossref, Medline, Google Scholar
80. George MS, Ketter TA, Gill DS, Haxby JV, Ungerleider LG, Herscovitch P, Post RM: Brain regions involved in recognizing facial emotion or identity: an oxygen-15 PET study. J Neuropsychiatry Clin Neurosci 1993; 5:384-394Crossref, Medline, Google Scholar
81. Lane RD, Fink GR, Chau PM, Dolan RJ: Neural activation during selective attention to subjective emotional responses. Neuroreport 1997; 8:3969-3972Crossref, Medline, Google Scholar
82. Pardo JV, Pardo PJ, Raichle ME: Neural correlates of self-induced dysphoria. Am J Psychiatry 1993; 150:713-719Link, Google Scholar
83. Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC: Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997; 277:968-971Crossref, Medline, Google Scholar
84. Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ: Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport 2000; 11:1739-1744Crossref, Medline, Google Scholar
85. Francis S, Rolls ET, Bowtell R, McGlone F, O’Doherty J, Browning A, Clare S, Smith E: The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 1999; 10:453-459Crossref, Medline, Google Scholar
86. Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H: Neural structures associated with recognition of facial expressions of basic emotions. Proc R Soc Lond B Biol Sci 1998; 265:1927-1931Crossref, Medline, Google Scholar
87. Blair RJR, Morris JS, Frith CD, Perrett DI, Dolan RJ: Dissociable neural responses to facial expressions of sadness and anger. Brain 1999; 122:883-893Crossref, Medline, Google Scholar
88. Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL: A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J Cogn Neurosci 1997; 9:835-847Crossref, Medline, Google Scholar
89. Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JDE: Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 1998; 95:14494-14499Crossref, Medline, Google Scholar
90. Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD: Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 1998; 280:747-749Crossref, Medline, Google Scholar
91. Kiehl KA, Liddle PF, Hopfinger JB: Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology 2000; 37:216-223Crossref, Medline, Google Scholar
92. Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Andrew C, Bullmore ET: Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev 2000; 24:13-19Crossref, Medline, Google Scholar
93. Krams M, Rushworth MFS, Deiber MP, Frackowiak RSJ, Passingham RE: The preparation, execution and suppression of copied movements in the human brain. Exp Brain Res 1998; 120:386-398Crossref, Medline, Google Scholar
94. Carter CS, Macdonald AM, Botvinickk M, Ross LL, Stenger VA, Noll D, Cohen JD: Parsing executive processes: strategic vs evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 2000; 97:1944-1948Crossref, Medline, Google Scholar
95. Goldstein RZ, Volkow ND, Wang G-J, Fowler JS, Rajaram S: Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport 2001; 12:2595-2599Crossref, Medline, Google Scholar
96. Nobre AC, Coull JT, Frith CD, Mesulam MM: Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nat Neurosci 1999; 2:11-12Crossref, Medline, Google Scholar
97. Hugdahl K, Berardi A, Thompson WL, Kosslyn SM, Macy R, Baker DP, Alpert NM, Ledoux JE: Brain mechanisms in human classical conditioning: a PET blood flow study. Neuroreport 1995; 6:1723-1728Crossref, Medline, Google Scholar
98. Chua P, Krams M, Toni I, Passingham R, Dolan R: A functional anatomy of anticipatory anxiety. Neuroimage 1999; 9:563-571Crossref, Medline, Google Scholar
99. Robbins TW, Joyce EM, Sahakian BJ: Neuropsychology and neuroimaging of affective disorders, in Handbook of Affective Disorders. Edited by Paykel ES. London, Churchill Livingston, 1992, pp 289-310Google Scholar
100. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675-682Abstract, Google Scholar
101. Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ: Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol Med 1998; 28:559-571Crossref, Medline, Google Scholar
102. Teneback CC, Nahas Z, Speer AM, Molloy M, Stallings LE, Spicer KM, Risch SC, George MS: Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci 1999; 11:426-435Medline, Google Scholar
103. Hickie I, Ward P, Scott E, Haindl W, Walker B, Dixon J, Turner K: Neo-striatal rCBF correlates of psychomotor slowing in patients with major depression. Psychiatry Res 1999; 92:75-81Crossref, Medline, Google Scholar



