Catechol O-Methyltransferase, Serotonin Transporter, and Tryptophan Hydroxylase Gene Polymorphisms in Bipolar Disorder Patients With and Without Comorbid Panic Disorder
Abstract
OBJECTIVE: Genetic epidemiologic and clinical data suggest that comorbid panic disorder may define a subtype of bipolar disorder. Comorbid panic disorder might thereby influence the strength of association between bipolar disorder and genes that have been implicated in bipolar disorder on the basis of their function in monoamine neurotransmission and previously reported linkage results. Polymorphic markers at catechol O-methyltransferase (COMT), serotonin transporter (5-HTT), and tryptophan hydroxylase (TPH) genes were analyzed in a case-control association study of bipolar disorder patients with or without lifetime panic disorder. METHOD: Unrelated subjects of Italian descent meeting DSM-III-R criteria for lifetime bipolar disorder (N=111), with (N=49) or without (N=62) comorbid lifetime panic disorder, were compared to 127 healthy subjects. DNA was extracted from blood leukocytes. The frequencies of COMT Val158Met, 5-HTTLPR, and TPH IVS7+218C>A polymorphisms were determined. Genotype and allele frequency comparisons between affected (bipolar disorder, bipolar disorder without panic disorder, or bipolar disorder with panic disorder) and unaffected individuals were carried out with chi-square tests or Fisher’s exact tests. RESULTS: Relative to the comparison subjects, subjects with bipolar disorder without panic disorder, but not those with comorbid bipolar disorder and panic disorder, showed significantly higher frequencies of the COMT Met158 and the short 5-HTTLPR alleles and genotypes. The differences in the frequencies of the TPH IVS7+218A alleles and genotypes approached statistical significance. CONCLUSIONS: The findings support the hypothesis that comorbid panic disorder identifies a genetic subtype of bipolar disorder and suggest a role for COMT and 5-HTT in vulnerability to these disorders.
Family, twin, and adoption studies show that bipolar disorder is highly heritable (1). Nevertheless, efforts to identify susceptibility genes for bipolar disorder have led to largely inconclusive results (2). The lack of conclusive findings may be attributable in part to the fact that bipolar disorder is a common disease with complex inheritance (3). An obstacle to identification of genes for bipolar disorder and other heritable psychiatric disorders is the lack of resolution of the affected phenotype, leading to a lumping together of etiologically heterogeneous disorders (4). Although psychiatric disorders probably represent final common pathways for different causal processes (4) and it is unlikely that improved definition of the phenotype will create a Mendelian disorder from a complex psychiatric disorder, insufficient progress has been made in the refinement of the psychiatric phenotype for the purpose of reducing genetic heterogeneity (5).
There is evidence from genetic epidemiologic data and even some linkage data that bipolar disorder that is comorbid with panic disorder may define a genetic subtype of bipolar disorder. In two family studies, De Paulo and co-workers (6, 7) found that panic disorder had a significantly higher prevalence in first-degree relatives with bipolar disorder in the family of probands with comorbid bipolar disorder and panic disorder, compared to relatives of bipolar disorder probands without panic disorder. The same authors reanalyzed their bipolar disorder linkage data in a data set that showed linkage to chromosome 18q and that was stratified for the presence or absence of familial comorbid panic disorder (8). They found stronger statistical evidence for linkage to 18q in the families of probands with comorbid bipolar disorder and panic disorder than in those with bipolar disorder without panic disorder (8). These findings are consistent with the hypothesis that panic disorder is an index of genetic heterogeneity in bipolar disorder.
We hypothesized that comorbidity for panic disorder may also influence the strength of associations between bipolar disorder and three candidate genes implicated by their role in monoamine neurotransmission. If comorbid panic disorder identifies an etiologically distinctive bipolar disorder subgroup, we expected that one or more of these genetic associations would be strengthened when individuals with bipolar disorder were stratified by the presence of panic disorder. The three candidate genes we considered were catechol O-methyltransferase (COMT) (9, 10) serotonin transporter (5-HTT) promoter (11–13), and tryptophan hydroxylase (TPH) (14).
Genetic variation in COMT, which metabolizes dopamine, norepinephrine, and epinephrine, could be important in modulating the diverse functions in which these monoamines are involved. The COMT Val158Met polymorphism is common, with a rare allele frequency of about 0.4; subjects with the low-activity Met158 allele have a three- to fourfold lower level of enzymatic activity and a lower level of thermal stability than subjects without this allele (15, 16). Significant association with bipolar disorder has been reported for the low-activity allele Met158 in the Han Chinese population (9) and in rapid cycling bipolar disorder patients (10). Furthermore, there is substantial evidence from linkage studies for a susceptibility locus for bipolar disorder in the chromosome 22 q11–13 region, which includes the COMT gene (17, 18).
A functional 44-base-pair insertion/deletion polymorphism was detected in the promoter region of the serotonin transporter (5-HTT) gene (19). The short allele (484 base pairs) of the 5-HTT gene-linked polymorphic region (5-HTTLPR) is associated with a lower level of transcriptional efficiency of the 5-HTT promoter in vitro (19), and genotype-determined differences in the in vivo expression of 5-HTT have also been reported (20). Two independent investigations (11, 12) and a meta-analysis (13) showed weak evidence of genetic association between the 5-HTTLPR low-activity allele and bipolar disorder.
The TPH gene codes for the rate-limiting enzyme in the synthesis of serotonin. Two relatively common bi-allelic polymorphisms have been reported in the TPH intron 7: TPH IVS7+779C>A (19) and TPH IVS7+218C>A (20). Although no common functional polymorphisms of TPH are yet known, these two polymorphisms are in strong linkage disequilibrium (21, 22). Association between the TPH IVS7+218C>A variant and bipolar disorder has been reported by Bellivier and coworkers (14).
In this report we attempt to clarify the relationship of these three monoaminergic candidate genes to bipolar disorder by using comorbid panic disorder as a grouping variable to better specify the bipolar disorder phenotype and, potentially, to reduce heterogeneity in the etiology of bipolar disorder.
Method
Subjects
Unrelated subjects with a lifetime diagnosis of bipolar disorder (N=111) were recruited from inpatients consecutively admitted at the Department of Psychiatry, Neurobiology, Pharmacology, and Biotechnology of the University of Pisa, Italy. Bipolar disorder patients were stratified according to the presence (N=49) or absence (N=62) of lifetime comorbid panic disorder. Subjects with no lifetime history of psychiatric disorders (N=127) were recruited among local blood donors (N=81) and from the administrative and hospital staff of the University of Pisa (N=46). All subjects included in the study were Caucasians of Italian descent. Exclusion criteria were current and lifetime organic brain syndromes, mental retardation, and major medical illnesses that could justify a diagnosis of a secondary mood or anxiety disorder. The patients and comparison subjects were assessed for psychiatric diagnoses according to DSM-III-R criteria by using the Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P) (23) and the Structured Clinical Interview for DSM-III-R—Non-Patient Edition (24), respectively. The SCID-P interviews were performed by the principal investigator (A.R.) and two research psychiatrists (S.B. or C.G.). The interviews were reviewed for accuracy by the program coordinator (L.D.). Interrater reliability was assessed in 24 interviews with eight patients. The kappa coefficients of agreement between raters were 1.00 for bipolar disorder and 0.97 for panic disorder. Information on family history of psychiatric disorders was obtained from probands by using the Family Informant Schedule and Criteria (25). Demographic and clinical data for the patients and comparison subjects are reported in Table 1. After a complete description of the study to the subjects, written informed consent was obtained. The study was approved by the Ethical Committee of the University of Pisa, the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program Institutional Review Board, and the National Institutes of Health Office of Protection from Research Risks.
Genotyping
DNA was extracted from blood leukocytes by using a commercial DNA extraction kit (Gentra Systems, Minneapolis, Minn.).
Genotyping of the COMT Val158Met variant was carried out with a polymerase chain reaction (PCR)-based restriction fragment length polymorphism analysis by using the primers 5′-CTCATCACCATCGAGATCAA-3′ and 5′-CCAGGTCTGAAACGGGTCA-3′. Conditions for DNA amplification were 30 cycles at 95°C for 15 seconds, 54°C for 20 seconds, and 72°C for 30 seconds. PCR products (109 base pairs) were digested by using the enzyme NlaIII, with the COMT Val allele cut into fragments of 86 base pairs and 23 base pairs and the COMT Met allele cut into fragments of 68 base pairs, 23 base pairs, and 18 base pairs. The 5-HTTLPR promoter 44-bases insertion/deletion polymorphism was genotyped as described by Heils et al. (19). The TPH IVS7+ 218C>A genotypes were determined according to procedures described by Nielsen et al. (22).
Statistical Analyses
Univariate comparisons of demographic and clinical data between groups were conducted with the independent sample t test for continuous variables or the chi-square test for categorical variables.
As is conventional in association studies, we present genotype frequencies along with allele frequencies. Whatever the convention, it is important to note that there are strong reasons not to use allele frequencies, particularly for biallelic markers (26). For the case-control genetic comparisons, differences in genotype and allele frequencies between affected (bipolar disorder, bipolar disorder without panic disorder, and bipolar disorder with panic disorder patients) and unaffected individuals were evaluated by using the chi-square test or Fisher’s exact test, where appropriate. Yates’s correction was applied in two-by-two tables. Since we compared genotypic and allelic distributions of three independent polymorphic markers between healthy comparison subjects and three diagnostic groups of patients, a Bonferroni correction was applied to correct for multiple testing (level of significance was set to alpha<0.05/3=0.017). All statistical tests were performed with the statistical package SPSS, version 10.0. Power to detect association was estimated using nQUERY, version 1.0.
Results
Demographic and Clinical Data
The patients and the comparison subjects did not differ in gender distribution. The patients were an average of about 3 years older than the comparison subjects at the time of the interview (mean age=33.8 years, SD=10.9, versus mean=30.9 years, SD=7.4) (t=2.3, df=236, p=0.02). As shown in Table 1, the age at the time of the interview, age at the onset of bipolar disorder, and gender distribution were similar in the group with bipolar disorder without panic disorder and the group with comorbid bipolar disorder and panic disorder. The percentage of subjects with a family history of mood disorders did not differ between the bipolar disorder groups with and without panic disorder. As expected, anxiety disorders were significantly more frequent in the families of patients with comorbid bipolar disorder and panic disorder than in the families of the bipolar disorder patients without panic disorder. The patients with comorbid bipolar disorder and panic disorder had higher rates of all co-occurring axis I disorders and suicidality but a lower rate of psychotic symptoms, compared to the bipolar disorder patients without panic disorder, although all of these comparisons were nonsignificant.
COMT Val158Met polymorphism
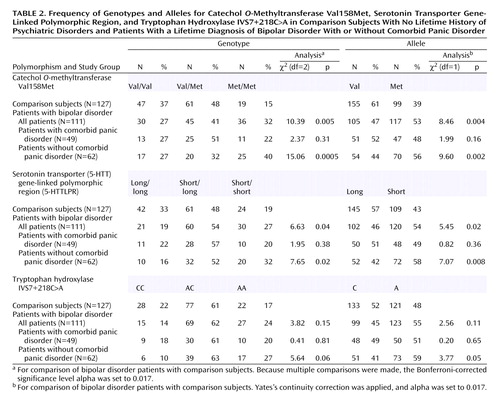
Genotypes and allele frequencies for the COMT Val158Met variant in the overall group of bipolar disorder patients, in the bipolar disorder patients with and without panic disorder, and in the comparison subjects are shown in Table 2. Genotype frequencies in the patient groups and the comparison group were in Hardy-Weinberg equilibrium. The frequency of the low-activity Met158 allele in the comparison subjects (39%) was similar to the allele frequency reported in other Caucasian populations (27–29). However, the frequency of the Met158 allele (and corresponding genotypes) was higher for the bipolar disorder patients (53%) than for the comparison subjects, even after Bonferroni correction (χ2=8.46, df=1, p=0.004), and this effect was strongest for the bipolar disorder patients without panic disorder (56%) (χ2=9.60, df=1, p=0.002). Forty percent of the bipolar disorder patients without panic disorder were low-activity Met158/Met158 homozygotes, compared to the expected frequency of about 16% in the general population. Higher frequencies of the COMT Met158/Met158 genotype and of the COMT Met158 allele were observed in the patients with comorbid bipolar disorder and panic disorder (48%) than in the comparison subjects, but the difference was not significant (χ2=1.99, df=1, p=0.16).
5-HTTLPR Polymorphism
The 5-HTTLPR genotype and allele frequencies in the overall group of bipolar disorder patients, the bipolar disorder patients with and without panic disorder, and the comparison subjects are reported in Table 2. The genotype frequencies did not deviate significantly from the Hardy-Weinberg equilibrium. The frequency of the short allele in the comparison group (43%) was again similar to the allele frequency reported in other Caucasian populations (11, 13, 30, 31). The overall group of bipolar disorder patients had a higher frequency of the short allele (and associated genotypes) (54%) than the comparison group (χ2=5.45, df=1, p=0.02), and this effect was again stronger in the group of bipolar disorder patients without panic disorder (58%) (χ2=7.07, df=1, p=0.008). A higher frequency of the short allele was detected in the group with bipolar disorder and panic disorder (49%) than in the comparison group, but the difference was not significant (χ2=0.82, df=1, p=0.36).
TPH IVS7+218C>A Polymorphism
The TPH IVS7+218C>A genotype and allele frequencies in the overall group of bipolar disorder patients, the bipolar disorder patients with and without panic disorder, and the comparison subjects are reported in Table 2. The genotype frequencies were in Hardy-Weinberg equilibrium. The IVS7+218A allele frequency in the comparison group (48%) was higher than that reported in previous studies with groups of French (37%) (14), English (39%) (32), Finnish (43%) (22), and Swedish (43%) (22) comparison subjects. The differences in allele and genotype frequencies between the comparison group and the bipolar disorder groups (overall bipolar disorder group and bipolar disorder groups with and without panic disorder) did not reach significance.
Epistasis
We tested for interactions among COMT Val158Met, 5-HTTLPR, and TPH IVS7+218C>A polymorphisms in a logistic regression model and found no evidence of epistatic effects (data not shown).
Discussion
Using a case-control association strategy, we found evidence that panic disorder is a marker of genetic heterogeneity in bipolar disorder, strengthening already significant associations of functional COMT and serotonin transporter polymorphisms with bipolar disorder. Our findings indicate strong genotypic and allelic association of two independent variants of COMT and 5-HTT genes with bipolar disorder in the absence of comorbid lifetime panic disorder. These associations remained statistically significant even after Bonferroni correction. Associations of these variants with comorbid bipolar disorder and panic disorder were more modest and did not withstand correction for multiple comparisons. Although clinical variables other than panic disorder (e.g., age at onset of bipolar disorder, diagnoses comorbid with bipolar disorder, suicidality, and presence of psychotic symptoms) could in theory mediate an association between bipolar disorder and the genes analyzed, we decided not to analyze the relationship of these clinical characteristics and the genotype and allele distributions to avoid inflation of type I error. In agreement with recent family data (6, 7), we also found that anxiety disorders were significantly more prevalent in families of subjects with comorbid bipolar disorder and panic disorder than in those of subjects with bipolar disorder without panic disorder. This finding should be considered with caution, as the family information was collected from probands only (33). However, it is consistent with a difference in familial vulnerability between these two bipolar disorder phenotypes.
To our knowledge, this is the first use of panic disorder as a grouping variable in a case-control association study of bipolar disorder. Taken together, our findings provide further support for the genetic epidemiologic (6, 7) and linkage (8) evidence suggesting that comorbid panic disorder is a marker for a distinct genetic subtype of bipolar disorder. Epidemiological and clinical findings also raise suspicion that the comorbidity between the two disorders is the manifestation of a specific phenotype. Epidemiological studies suggest that comorbidity rates between bipolar disorder and panic disorder were higher than could be expected by chance alone. In the Epidemiologic Catchment Area study in the United States, the lifetime prevalence of panic disorder among subjects with bipolar disorder was 20.8%, 26 times higher than in subjects without any other axis I disorder (0.8%) and 2.1 times higher than in individuals with unipolar depression (10.0%) (34). In agreement with these findings, the National Comorbidity Survey, a general population survey of DSM-III-R disorders in the United States, reported that 33.1% of subjects with bipolar disorder had comorbid panic disorder (35) and were at greater risk for panic disorder than subjects with unipolar depression (36). The lifetime prevalence of panic disorder among bipolar disorder subjects in clinical study groups is even higher, as high as 62.5% (37–40). Clinically, it has been suggested that panic disorder and high anxiety levels in the context of bipolar disorder predict greater severity, poorer prognosis, and resistance to pharmacological treatments (38, 41, 42).
On the basis of the significant results reported here, bipolar disorder without panic disorder may represent a more homogeneous form of the illness, genetically distinct from bipolar disorder with panic disorder and more strongly related to the function of COMT Val158Met and 5-HTTLPR. These variants may influence specific clinical features of bipolar disorder independent of those affecting liability (43). Therefore, the association of both of these polymorphisms with a genetic subtype of bipolar disorder, such as bipolar disorder without panic disorder, may be better understood as a relationship of alleles to a modifying trait for various psychiatric diseases, rather than a one-to-one causative relationship between functional alleles and a particular psychiatric disease (43).
The success of the clinical subgrouping in this study may partly explain the inconsistent results for the association of COMT and 5-HTT variants with the broadly defined bipolar disorder phenotype, since it is likely that the frequency of panic disorder differs in different groups of bipolar disorder patients. Thus far, several studies of the COMT Val158Met polymorphism in bipolar disorder have had negative findings (27–29, 44, 45), although a positive association was found between the low-activity Met158 allele and bipolar disorder in a Han Chinese population (9) and in a group of rapid cycling bipolar disorder patients (10). The COMT gene falls within the region of chromosome 22q11, which has been indicated among possible susceptibility loci for bipolar disorder in linkage studies (17, 18). On the basis of the earlier family (6, 7) and linkage (8) findings and the current results, we suggest that stratification of chromosome 22 linkage data by familial comorbid panic disorder could be useful for increasing the resolution of linkage analysis. As for 5-HTTLPR, a number of studies failed to find a role for this polymorphism in bipolar disorder (13, 30, 31, 46–48), although two independent investigations (11, 12) and a meta-analysis (13) found weak evidence for a genetic association between the 5-HTTLPR low-activity allele and bipolar disorder. Finally, it is also possible that the association between the TPH IVS7+218C>A variant and bipolar disorder reported by Bellivier and coworkers (14), while not replicated elsewhere (32, 48–50), could be strengthened and eventually validated with the aid of clinical subgrouping.
The strength of our conclusions may be mitigated by several methodological limitations. Although the size of the study group was fairly large in comparison to those in many association studies in neuropsychiatry, statistical power to detect potentially important effects may have been limited. For example, for a susceptibility allele present in comparison subjects with a frequency of 0.4, we could detect genotypic relative risks of approximately 2.7 under a dominant model and 2.8 under a recessive model with 80% power (assuming two-tailed alpha=0.05) for the critical comparisons between the group with comorbid bipolar disorder and panic disorder and the comparison group or between the bipolar disorder patients without panic disorder and the comparison group. Therefore, further studies with larger study groups are needed to draw definitive conclusions.
A possible limitation in case-control association studies is the risk of spurious associations as a result of population admixture (51). However, this objection is less likely in the present study, as our study group was composed of subjects of Italian descent for generations and because the Italian population is relatively homogeneous (52). We also note that the COMT and HTTLPR allele frequencies observed in the Italian comparison subjects are similar to the allele frequencies found in diverse Caucasian populations.
Despite these limitations, our report provides additional evidence for a role for two loci in bipolar disorder and for the existence of subgroups of bipolar disorder indexed by comorbidity with panic disorder. Future research involving larger study groups will be required to determine whether a clinical subtype of bipolar disorder exists and, if so, whether it represents a more homogeneous phenotype for genetic studies. To finally exclude cryptic population stratification as the source of the observed association, replication studies with a family-based design and control loci to formally evaluate stratification may be employed.
 |
 |
Received Dec. 18, 2000; revision received June 4, 2001; accepted July 13, 2001. From the Department of Psychiatry, Neurobiology, Pharmacology, and Biotechnology, University of Pisa; the Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Rockville, Md.; and the Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, Va. Address reprint requests to Dr. Rotondo, Department of Psychiatry, Neurobiology, Pharmacology, and Biotechnology, University of Pisa, via Roma 67, 56100 Pisa, Italy; [email protected] (e-mail). Supported in part by the IDEA Association (Institute for the Treatment and Prevention of Depression and Anxiety), Milan (Dr. Rotondo). The authors thank Cynthia Bulik, Ph.D., for critical reading of the manuscript and Cara Sokolsky and Maria Rosa Doria for technical assistance.
1. Merikangas KR, Kupfer DJ: Mood disorders: genetic aspects, in Comprehensive Textbook of Psychiatry, 6th ed. Edited by Kaplan HI, Sadock BJ. Baltimore, Williams & Wilkins, 1995, pp 1102-1116Google Scholar
2. Berrettini W: Progress and pitfalls: bipolar molecular linkage studies. J Affect Disord 1998; 50:289-297Google Scholar
3. Risch N, Merikangas KR: Linkage studies of psychiatric disorders. Eur Arch Psychiatry Clin Neurosci 1993; 243:143-149Crossref, Medline, Google Scholar
4. Klein D, Riso L: Psychiatric disorders: problems of boundaries and comorbidity, in Basic Issues in Psychopathology. Edited by Costello CG. New York, Guilford, 1993, pp 19-66Google Scholar
5. Smoller JW, Tsuang MT: Panic and phobic anxiety: defining phenotypes for genetic science. Am J Psychiatry 1998; 155:1152-1162Link, Google Scholar
6. Cooper J, DePaulo J: Familial bipolar disorder and panic disorder in the National Institute of Mental Health Genetic Initiative Bipolar Disorder Study (abstract). Bipolar Disord 1999; 1(suppl 1):28Google Scholar
7. MacKinnon DF, McMahon FJ, Simpson SG, McInnis MG, DePaulo JR: Panic disorder with familial bipolar disorder. Biol Psychiatry 1997; 42:90-95Crossref, Medline, Google Scholar
8. MacKinnon DF, Xu J, McMahon FJ, Simpson SG, Stine OC, McInnis MG, DePaulo JR: Bipolar disorder and panic disorder in families: an analysis of chromosome 18 data. Am J Psychiatry 1998; 155:829-831Link, Google Scholar
9. Li T, Vallada H, Curtis D, Arranz M, Xu K, Guiqing C, Deng H, Liu H, Murray R, Liu X, Collier DA: Catechol-O-methyltransferase Val158Met polymorphism: frequency analysis in Han Chinese subjects and allelic association of the low activity allele with bipolar affective disorder. Pharmacogenetics 1997; 7:349-353Crossref, Medline, Google Scholar
10. Kirov G, Murphy K, Aranz M, Jones I, McCandles F, Kunugi H, Murray R, McGuffin P, Collier D, Owen M, Craddock N: Low activity of catechol-O-methyltransferase gene associated with rapid cycling bipolar disorder. Mol Psychiatry 1998; 3:342-345Crossref, Medline, Google Scholar
11. Collier D, Stober G, Heils A, Catalano M, Di Bella D, Arranz M, Murray R, Vallada H, Bengel D, Muller C, Roberts G, Smeraldi E, Kirov G, Sham P, Lesch K: A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry 1996; 1:453-460Medline, Google Scholar
12. Bellivier F, Henry C, Szoke A, Schurhoff F, Nosten-Bertrand M, Feingold J, Launay J, Leboyer M, Laplanche J: Serotonin transporter gene polymorphisms in patients with unipolar or bipolar depression. Neurosci Lett 1998; 255:143-146Crossref, Medline, Google Scholar
13. Furlong R, Ho L, Walsh C, Rubinsztein J, Jain I, Paykel E, Easton D, Rubinsztein D: Analysis and meta-analysis of two serotonin transporter gene polymorphisms in bipolar and unipolar affective disorders. Am J Med Genet 1998; 81:58-63Crossref, Medline, Google Scholar
14. Bellivier F, Leboyer M, Courtet P, Buresi C, Beaufils B, Samolyk D, Allilaire JF, Feingold J, Mallet J, Malafosse A: Association between the tryptophan hydroxylase gene and manic-depressive illness. Arch Gen Psychiatry 1998; 55:33-37Crossref, Medline, Google Scholar
15. Spielman RS, Weinshilboum RM: Genetics of red cell COMT activity: analysis of thermal stability and family data. Am J Med Genet 1981; 10:279-290Crossref, Medline, Google Scholar
16. Aksoy S, Klener J, Weinshilboum R: Catechol-O-methyltransferase pharmacogenetics: photoaffinity labelling and Western blot analysis of human liver samples. Pharmacogenetics 1993; 3:116-122Crossref, Medline, Google Scholar
17. Kelsoe J, Spence M, Loetscher E, Monserrat F, Sadovnick A, Remick R, Flodman P, Khristich J, Mroczkowski-Parker Z, Brown J, Masse D, Ungerleider S, Rapaport M, Wishart W, Luebbert H: A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc Natl Acad Sci 2001; 98:585-590Crossref, Medline, Google Scholar
18. Berrettini W: Susceptibility loci for bipolar disorder: overlap with inherited vulnerability to schizophrenia. Biol Psychiatry 2000; 47:245-251Crossref, Medline, Google Scholar
19. Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch K: Allelic variation of human serotonin transporter gene expression. J Neurochem 1996; 66:2621-2624Crossref, Medline, Google Scholar
20. Heinz A, Jones D, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger D: A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry 2000; 47:643-649Crossref, Medline, Google Scholar
21. Nielsen DA, Dean M, Goldman D: Genetic mapping of the human tryptophan hydroxylase gene on chromosome 11, using an intronic conformational polymorphism. Am J Hum Genet 1992; 51:1366-1371Medline, Google Scholar
22. Nielsen DA, Jenkins GL, Stefanisko KM, Jefferson KK, Goldman D: Sequence, splice site and population frequency distribution analyses of the polymorphic human tryptophan hydroxylase intron 7. Mol Brain Res 1997; 45:145-148Crossref, Medline, Google Scholar
23. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1990Google Scholar
24. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
25. Mannuzza S, Fyer AJ, Endicott J, Klein DF: Family Informant Schedule and Criteria (FISC). New York, New York State Psychiatric Institute, Anxiety Disorders Clinic, 1985Google Scholar
26. Sasieni PD: From genotypes to genes: doubling the sample size. Biometrics 1997; 53:1253-1261Crossref, Medline, Google Scholar
27. Lachman H, Kelsoe J, Moreno L, Katz S, Papolos D: Lack of association of catechol-O-methyltransferase (COMT) functional polymorphism in bipolar affective disorder. Psychiatr Genet 1997; 7:1-17Crossref, Medline, Google Scholar
28. Gutiérrez B, Bertranpetit J, Guillamat R, Vallès V, Arranz MJ, Kerwin R, Fañanás L: Association analysis of the catechol O-methyltransferase gene and bipolar affective disorder. Am J Psychiatry 1997; 154:113-115Link, Google Scholar
29. Biomed European Bipolar Collaborative Group: No association between bipolar disorder and alleles at a functional polymorphism in the COMT gene. Br J Psychiatry 1997; 170:526-528Crossref, Medline, Google Scholar
30. Rees M, Norton N, Jones I, McCandless F, Scourfield J, Holmans P, Moorhead S, Feldman E, Sadler S, Cole T, Redman K, Farmer A, McGuffin P, Owen MJ, Craddock N: Association studies of bipolar disorder at the human serotonin transporter gene (hSERT; 5HTT). Mol Psychiatry 1997; 2:398-402Crossref, Medline, Google Scholar
31. Gutiérrez B, Arranz M, Collier D, Vallès V, Guillamat R, Bertranpetit J, Murray R, Fañanás L: Serotonin transporter gene and risk for bipolar affective disorder: an association study in a Spanish population. Biol Psychiatry 1998; 43:843-847Crossref, Medline, Google Scholar
32. McQuillin A, Lawrence J, Kalsi G, Gurling H, Curtis D: No allelic association between bipolar affective disorder and the tryptophan hydroxylase gene. Arch Gen Psychiatry 1999; 56:99-100Crossref, Medline, Google Scholar
33. Chapman TF, Mannuzza S, Klein DF, Fyer AJ: Effects of informant mental disorder on psychiatric family history data. Am J Psychiatry 1994; 151:574-579Link, Google Scholar
34. Chen Y-W, Dilsaver SC: Comorbidity of panic disorder in bipolar illness: evidence from the Epidemiologic Catchment Area survey. Am J Psychiatry 1995; 152:280-282Link, Google Scholar
35. Kessler R, Rubinow D, Holmes C, Abelson J, Zhao S: The epidemiology of DSM-III-R bipolar I disorder in a general population survey. Psychol Med 1997; 27:1079-1089Crossref, Medline, Google Scholar
36. Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler KS: Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8-19Crossref, Medline, Google Scholar
37. Strakowsky SM, Tohen M, Stoll AL, Faedda GL, Goodwin DC: Comorbidity in mania at first hospitalization. Am J Psychiatry 1992; 149:554-556Link, Google Scholar
38. Young LT, Cooke RG, Robb JC, Levitt AJ, Joffe RT: Anxious and non-anxious bipolar disorder. J Affect Disord 1993; 29:49-52Crossref, Medline, Google Scholar
39. Dilsalver SC, Chen YW, Swann AC, Shoaib A, Tsai-Dilsaver Y, Krajewski KJ: Suicidality, panic disorder and psychosis in bipolar depression, depressive-mania and pure-mania. Psychiatry Res 1997; 73:47-56Crossref, Medline, Google Scholar
40. Savino M, Perugi G, Simonini E, Soriani A, Cassano GB, Akiskal HS: Affective comorbidity in panic disorder: is there a bipolar connection? J Affect Disord 1993; 28:155-163Crossref, Medline, Google Scholar
41. Feske U, Frank E, Mallinger AG, Houck PR, Fagiolini A, Shear MK, Grochocinski VJ, Kupfer DJ: Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry 2000; 157:956-962Link, Google Scholar
42. Roy-Byrne P, Stang P, Wittchen H-S, Ustun B, Walters E, Kessler R: Lifetime panic-depression comorbidity in the National Comorbidity Survey: association with symptoms, impairment, course and help-seeking. Br J Psychiatry 2000; 176:229-235Crossref, Medline, Google Scholar
43. Owen M, Cardno A, O’Donovan M: Psychiatry genetics: back to the future. Mol Psychiatry 2000; 5:22-31Crossref, Medline, Google Scholar
44. Kirov G, Jones I, McCandless F, Craddock N, Owen M: Family-based association studies of bipolar disorder with candidate genes involved in dopamine neurotransmission: DBH, DAT1, COMT, DRD2, DRD3, and DRD5. Mol Psychiatry 1999; 4:558-565Crossref, Medline, Google Scholar
45. Kunugi H, Vallada H, Hoda F, Kirov G, Gill M, Aitchison K, Ball D, Arranz M, Murray R, Collier D: No evidence for an association of affective disorders with high- or low-activity of catechol-O-methyltransferase gene. Biol Psychiatry 1997; 42:282-285Crossref, Medline, Google Scholar
46. Esterling L, Yoshikawa T, Turner G, Badner JA, Bengel D, Gershon E, Berrettini W, Detera-Wadleigh S: Serotonin transporter (5-HTT) gene and bipolar affective disorder. Am J Med Genet 1998; 81:37-40Crossref, Medline, Google Scholar
47. Kunugi H, Hattori M, Kato T, Tatsumi M, Sakai T, Sasaki T, Hirose T, Nanko S: Serotonin transporter gene polymorphisms: ethnic difference and possible association with bipolar affective disorder. Mol Psychiatry 1997; 2:457-462Crossref, Medline, Google Scholar
48. Vincent JB, Masellis M, Lawrence J, Choi V, Gurling HMD, Parikh SV, Kennedy JL: Genetic association analysis of serotonin system genes in bipolar affective disorder. Am J Psychiatry 1999; 156:136-138Link, Google Scholar
49. Kirov G, Owen M, Jones I, McCandless F, Craddock N: Tryptophan hydroxylase gene and manic-depressive illness. Arch Gen Psychiatry 1999; 56:98-99Crossref, Medline, Google Scholar
50. Furlong R, Ho L, Rubinsztein J, Walsh C, Paykel E, Rubinsztein D: No association of the tryptophan hydroxylase gene with bipolar affective disorder, unipolar affective disorder, or suicidal behavior in major affective disorder. Am J Med Genet 1998; 81:245-247Crossref, Medline, Google Scholar
51. Owen MJ, Craddock N: Modern molecular genetic approaches to complex traits: implication for psychiatric disorders. Mol Psychiatr 1996; 1:21-26Medline, Google Scholar
52. Fuciarelli M, Vienna A, Paba E, Bastianini A, Sansonetti B, Capucci E, Destefano G: PI, GC, HP, and TF serum protein polymorphisms in Sienna, Tuscany, Italy, with a review of data for Italy. Am J Hum Biol 1997; 9:629-646Crossref, Google Scholar



