Multinuclear Magnetic Resonance Spectroscopy Studies of Brain Purines in Major Depression
Abstract
OBJECTIVE: Studies of depressed adults have shown abnormalities in cerebral energy metabolism, as noted by low brain levels of nucleoside triphosphate (NTP), which primarily represents adenosine triphosphate (ATP). This study was undertaken to determine whether proton magnetic resonance spectroscopy (1H MRS) measures of the low-field purine resonance, which arises primarily from adenosine phosphates, can be used to assess abnormalities in cerebral purine metabolism in depressed adults. METHOD: Data from 1H MRS and phosphorus-31 (31P) MRS were acquired for depressed and nondepressed comparison subjects. Intensities of the purine resonance, by 1H MRS (7.5–8.5 ppm), and of NTP, by 31P MRS, were determined. RESULTS: Purine resonance intensities did not differ on average between depressed patients and comparison subjects. However, purine levels were approximately 30% lower in female depressed subjects who subsequently responded to fluoxetine treatment than in those who did not respond. Beta-NTP was lower by 21% in responders than in nonresponders and was correlated with purine levels for the depressed subjects. CONCLUSIONS: Brain purine levels are low in female depressed patients who respond to treatment with fluoxetine, suggesting that response to treatment might be predicted by using 1H MRS. These observations also suggest that agents that increase brain adenosine levels may have antidepressant efficacy.
Major depression has been associated with low measures of both global and regional cerebral blood flow and glucose metabolism, indicating low cerebral activity, as assessed by using emission tomography (1, 2). Similarly, phosphorus-31 magnetic resonance spectroscopy (31P MRS) has been used to document lower than normal levels of beta- and total nucleoside triphosphate (NTP) in the basal ganglia (3), –16% and –6%, respectively, and the frontal lobes (4), –17% and –8%, respectively, of depressed adults.
In the brain, adenosine triphosphate (ATP) is present at a much higher concentration, on the order of 1.8 mM, than any other NTP (5). Previous 31P MRS results (3, 4) suggesting lower levels of ATP in depressed subjects, are somewhat surprising as the brain concentration of ATP is usually maintained at the expense of phosphocreatine, owing to the higher phosphate group transfer potential of phosphocreatine (6).
Of note, there also have been reports of lower levels of beta-NTP and total NTP, –10% and –7%, respectively, as assessed by using 31P MRS, in the cerebral cortex of patients with polysubstance abuse (7) and of lower beta-NTP levels, –11%, in the basal ganglia of patients with schizophrenia (8). These disorders have both been associated with sustained cerebral hypometabolism in the brain regions tested (9, 10).
Of the currently available methods for the assessment of brain adenosine metabolites, 31P MRS, which provides a direct measure of NTP levels, is the most studied (11). An important limitation of 31P MRS for assessing brain chemistry is the relatively low sensitivity of the method, approximately 5% that of hydrogen (1H, proton) MRS (12). Thus, at 1.5 T, typical phosphorus and proton MRS voxels are on the order of 25–100 cm3 and 1–10 cm3, respectively. Over the last several years, van Zijl and colleagues (13–15) have demonstrated that a 1H MRS resonance that arises from purines may be detected in the range of 7.8–8.8 ppm by using short echo times. This resonance develops primarily from adenosine phosphates, with a smaller contribution from N-acetylaspartate at 7.8–8.0 ppm. This resonance has not been studied extensively in human brain spectra, probably reflecting the short T2 relaxation time of the resonance and the fact that it lies on the opposite side of the water resonance (4.7 ppm) from the region where most resonances of interest are analyzed (1.4–3.6 ppm). In animals, at higher field strengths this resonance has been detected with echo times ranging from 14 to 20 msec, values that have not been available on clinical scanners until the last several years (13–15).
In this study, we reanalyzed short echo time (TE=30 msec) stimulated echo acquisition mode spectra acquired from the basal ganglia of depressed and healthy subjects, with a focus on the low-field purine resonance (16). In addition, because a subset of these subjects also participated in a 31P MRS study (3), it was possible to assess the relationship between the 1H MRS purine resonance and the 31P NTP resonance. Finally, as all of the depressed study subjects were enrolled in a standardized clinical trial of the antidepressant fluoxetine, 1H MRS purine measures were correlated with the clinical response to treatment.
We hypothesized that there would be lower levels of beta-NTP and purines in the depressed subjects than in the comparison group and that, within the depressed subgroup, the intensity of the beta-NTP and purine resonances would be correlated with severity of depressive symptoms.
Method
All aspects of the clinical research protocol were reviewed and approved by the institutional review boards of the Massachusetts General Hospital and McLean Hospital. After complete description of the study, written informed consent was obtained from all subjects.
Depressed subjects (N=38) were referred from the Depression Clinical and Research Program of the Massachusetts General Hospital, and comparison subjects (N=22) were recruited by newspaper advertisement (16). Depressed subjects were consecutively referred from the open phase of an NIMH-sponsored, double-blind study of fluoxetine treatment. At baseline, all depressed subjects met criteria for major depressive disorder, as determined by the Structured Clinical Interview for DSM-III-R (SCID) (17), and had baseline scores on the 17-item Hamilton Depression Rating Scale (18) of 16 or greater. Both of these instruments were administered by trained raters. Interrater reliability was assessed at kappa >0.80 for both instruments (19). Of the 38 depressed subjects who entered this study, 20 had no prior history of psychotropic medication. The remaining subjects had been treated with either fluoxetine or a tricyclic antidepressant in the past. These subjects had been medication free for either 1–3 weeks (N=9) or more than 1 year (N=9) before study entry. The exclusion criteria for participation in this study included history of neurological illness, serious medical illness, substance abuse, or claustrophobia; age less than 18 or greater than 60 years; or the presence of a ferrous implant or pacemaker.
The 22 comparison subjects were recruited by advertisement in local newspapers. After telephone screening, all comparison subjects were administered the SCID by the same raters. For inclusion in this study, the comparison subjects could not have any axis I diagnosis. Apart from this, the exclusion criteria were the same as for the depressed subjects.
The depressed subgroup participated in an antidepressant trial consisting of 8 weeks of fluoxetine treatment, 20 mg/day. Hamilton depression scale scores were determined at baseline and at the 8-week endpoint for all depressed subjects. Of the 38 depressed subjects entering the study, 36 subjects completed it.
All 60 subjects completed the baseline 1H MRS protocol, while 12 depressed and 17 comparison subjects completed the 31P MRS study. All subjects completing the 31P MRS examination also participated in the 1H MRS study, and both MRS assessments were completed during the same scanning session. All of the magnetic resonance (MR) spectra were obtained by using a GE Signa 1.5-T MR scanner (4.8 operating system). Proton spectra were acquired from an 8-cm3 voxel centered on the left caudate and putamen. The stimulated echo acquisition mode parameters included TR=2 sec, TE=30 msec, 256 averages, 1024 data points, and 2500-Hz spectral width. 31P spectra were acquired from a 45-cm3 volume encompassing the bilateral basal ganglia. The 8-cm3 proton MRS voxel was entirely included within the 45-cm3 phosphorus MRS voxel for all subjects completing both examinations. Acquisition parameters for image-selected in vivo spectroscopy included TR=3 seconds, flip angle=90°, acquisition delay=350 msec, 512 averages, 1024 data points, and 2500-Hz spectral width.
The spectra were analyzed by using SA/GE (General Electric Medical Systems, Milwaukee), as previously described (3, 16), with the analysts (A.M.P., F.H.) blind to clinical data. The intensity of the purine resonance was determined by integration of the 7.5–8.5 ppm region (Figure 1 and Figure 2) separately normalized to the intensity of the resonances for N-acetylaspartate (2.0 ppm), creatine plus phosphocreatine (3.0 ppm), and cytosolic choline (primarily phosphocholine and glycerophosphocholine [20]) (3.2 ppm). Ratios of the purine resonances to N-acetylaspartate, creatine plus phosphocreatine, and cytosolic choline were calculated as a volume normalization control; these were termed purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline, respectively. Proton voxel tissue content was determined by segmentation of T1-weighted images, as described by us elsewhere (16).
Beta-NTP and purine-metabolite ratios (purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline) were assessed independently by trained raters (C.M.M. for beta-NTP, A.M.P. and F.H. for ratios) blind to group status (depression versus comparison). After interrater reliability was checked, the average of two ratings was used in subsequent analyses.
The depressed group was separated into responder and nonresponder subgroups on the basis of the Hamilton depression scale scores. The criteria for responder status were determined a priori as achievement of a 50% or greater reduction in symptom severity on the Hamilton depression scale and a posttrial Hamilton depression scale score of 7 or less.
Contrasts on beta-NTP measurements and on purine/N-acetylaspartate, purine/choline, and purine/creatine-plus-phosphocreatine ratios between the comparison and depressed groups and between the responder and nonresponder depressed subgroups were carried out by using linear regression modeling methods with robust estimation of standard errors. Correlations between Hamilton depression scale severity scores and the beta-NTP, purine/N-acetylaspartate, purine/choline, and purine/creatine-plus-phosphocreatine measurements were assessed by using Spearman rank correlation and generalized least squares modeling methods. Statistical significance required two-tailed p<0.05. For contrasts not specified in advance, Bonferroni methods were used to correct for multiple comparisons (21).
Results
Of the 38 depressed subjects, 17 (45%) met the responder criteria, and the other 21 were designated nonresponders.
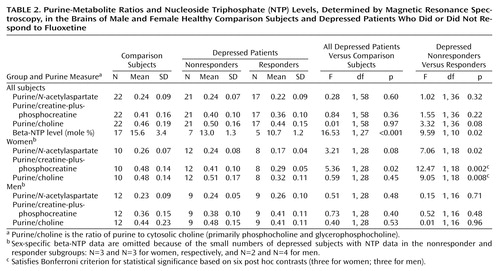
Figure 1 displays water-suppressed proton and phosphorus MR spectra obtained from a solution containing 20 mM ATP, with the pH adjusted to 7.0. These spectra display the low-field purine resonance at 7.9 and 8.2 ppm, as well as the phosphorus resonances arising from the gamma, alpha, and beta phosphates of ATP. As in vivo proton MRS metabolite line widths are generally much broader than those observed in solution, the purine resonance intensity was assessed by integrating the spectrum between 7.5 and 8.5 ppm.
Characteristics of the subjects in the proton study are summarized in Table 1. There were no sex or age differences among the three study subgroups: depressed responders (N=17), depressed nonresponders (N=21), and comparison subjects (N=22). The percentage of white matter did not differ among the subgroups. Baseline Hamilton depression scale scores did not differ between the responder and nonresponder subgroups.
Figure 2 displays the summed and averaged low-field 1H spectra for the comparison subjects, the depressed nonresponders, and the depressed responders. All of the spectra were scaled to the creatine-plus-phosphocreatine resonance (creatine-plus-phosphocreatine intensity=1) before summation.
Beta-NTP assessments and measures of intensity of the purine resonance, expressed as purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline, are summarized in Table 2. Estimates of interrater reliability, expressed as intraclass correlation coefficients for two independent raters (A.M.P., F.H.) evaluating 60 spectra, were 0.89, 0.92, and 0.84 for purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline, respectively. Because of the length of the combined examination, beta-NTP measurements were available for only 29 subjects (17 in the comparison subgroup, 12 in the depressed subgroup).
As hypothesized, estimated beta-NTP resonance was significantly lower, by 33%, in the depressed subjects than in the comparison subjects (Table 2, Figure 3). This finding is consistent with results previously reported by our group (3).
Contrary to our hypothesis, the intensity measures purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline did not differ significantly between the comparison and depressed groups. All three of these contrasts yielded p values exceeding 0.30 (Table 2).
Beta-NTP level was positively correlated with baseline Hamilton depression scale score. Similarly, purine/choline was significantly correlated with baseline Hamilton depression score. The correlations of the depression score with the purine/creatine-plus-phosphocreatine and purine/N-acetylaspartate ratios were in the same direction, although not meeting the significance criteria. These correlations were in the opposite direction from what was expected. Because prior research had shown beta-NTP levels to be lower in depressed subjects than in healthy comparison groups, we had expected a dose-related response, that is, low beta-NTP levels associated with high baseline Hamilton depression scale score.
Beta-NTP was positively correlated with the purine-metabolite ratios purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline. Within the depressed group, the coefficients for the Spearman rank correlations between beta-NTP and purine/N-acetylaspartate (rs=0.69, N=12, p=0.01) and between beta-NTP and purine/creatine-plus-phosphocreatine (rs=0.58, N=12, p=0.05) were statistically significant. For the association of beta-NTP and purine/choline, the Spearman correlation was also positive within the depressed group, but it did not reach statistical significance (rs=0.40, N=12, p=0.20). Among all subjects, these correlations were positive but nonsignificant (rs=0.14, 0.21, and 0.02 for the correlations of beta-NTP with purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline, respectively).
In contrasts of the depressed responders and nonresponders, there was a significant difference in beta-NTP levels, with the responder subgroup having a mean beta-NTP level about 21% lower than that of the nonresponders (10.7 versus 13.0) (Table 2). This difference was statistically significant (Table 2). The purine-metabolite ratios purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline did not differ between the depressed responder and nonresponder subgroups (Table 2).
While carrying out these analyses, we discovered differences between the comparison and depressed groups in the correlations of purine resonances with voxel white matter. Among the comparison subjects, the three purine-metabolite ratios were positively correlated with voxel white matter content (expressed as percentage of total voxel volume). The correlation coefficients were as follows: purine/choline: r=0.60, p=0.01; purine/creatine-plus-phosphocreatine: r=0.57, p=0.02; purine/N-acetylaspartate: r=0.46, p=0.06 (N=17 for all three correlations). In contrast, among the depressed subjects (N=33), the purine-metabolite ratios were uncorrelated with voxel white matter content (purine/choline: r=–0.02, p=0.89; purine/creatine-plus-phosphocreatine: r=–0.01, p=0.95; purine/N-acetylaspartate: r=0.03, p=0.85).
Also, differences between responders and nonresponders in the purine-metabolite ratios varied by sex. Among the women, responders had lower purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline ratios than nonresponders (by 29%, 29%, and 37%, respectively) (Table 2). These differences were determined post hoc, and so adjustment for multiple comparisons was necessary in the assessment of their statistical significance. After adjustment by the Bonferroni method, two of these contrasts remained statistically significant: purine/creatine-plus-phosphocreatine (F=12.47, df=1, 18, p=0.002, Bonferroni-adjusted p=0.02) and purine/choline (F=9.05, df=1, 18, p=0.008, Bonferroni-adjusted p=0.05) (Table 2). Among the men, there were no significant differences in the corresponding contrasts.
Within the depressed group, the women had significantly higher Hamilton depression scale scores at baseline than the men (women: mean=22.0, SD=4.2; men: mean=19.0, SD=3.6) (t=2.38, df=36, p=0.02). At endpoint, the mean score on the Hamilton scale for women (mean=12.4, SD=7.3) exceeded that for the men (mean=9.5, SD=6.8), but this difference did not attain statistical significance (t=1.48, df=35, p=0.15).
For women (N=15), beta-NTP was positively correlated with purine/N-acetylaspartate (rs=0.83, p<0.001) and purine/creatine-plus-phosphocreatine (rs=0.77, p<0.001). For the third purine ratio, purine/choline, the Spearman correlation coefficient was positive but not statistically significant (rs=0.37, p=0.17). In contrast, for men the three corresponding Spearman rank correlation coefficients were nonsignificant.
In summary, the study results are as follows:
1. Beta-NTP was lower, by 33%, in depressed subjects than in comparison subjects (p<0.001, Table 2).
2. Purine-metabolite ratios (purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline) did not differ between comparison and depressed subjects (Table 2).
3. For women within the depressed group, but not men, purine-metabolite ratios were significantly lower in the responder subgroup than in the nonresponder subgroup (by 29%, 29%, and 37% for purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline, respectively) (Table 2).
4. Beta-NTP was positively correlated with the Hamilton depression scale score at baseline.
5. Purine/choline was positively correlated with the baseline Hamilton depression scale score. For purine/creatine-plus-phosphocreatine and purine/N-acetylaspartate, this correlation was positive but nonsignificant.
6. Within the depressed group, the purine-metabolite ratios were positively correlated with beta-NTP resonance (rs=0.69, rs=0.58, and rs=0.40 for purine/N-acetylaspartate, purine/creatine-plus-phosphocreatine, and purine/choline, respectively).
7. In the comparison subjects, but not depressed subjects, the purine-metabolite ratios were positively correlated with voxel white matter content.
Discussion
To the best of our knowledge, this is the first report on purine-metabolite ratios in brain proton MR spectra from human subjects. If the findings of this study are sustained and extended, it may be that clinically relevant insights can be achieved through 1H MRS scanning of purines.
Because this was a retrospective study, optimal acquisition characteristics for detecting the purine resonance (14) could not be used. The use of shorter echo times, higher field strengths, and alternative methods of water suppression would all be expected to substantially increase the relative intensity of the purine resonance. In addition, application of spectroscopic imaging methods would allow the intensity of this metabolite line to be assessed across multiple brain regions. Nonetheless, despite these limitations, the present results suggest that lower brain purine intensities may be observed in treatment-responsive depressed subjects, at least among women. More generally, these observations suggest that 1H MRS may provide clinically relevant insights into cerebral metabolism with a substantial increase in sensitivity and regional resolution relative to 31P MRS measures. It is also possible that MRS assessments of other brain regions, such as the dorsolateral prefrontal cortex, may provide complementary information. For example, recent reports (22, 23) have emphasized that executive dysfunction is associated with poor response to treatment for major depression.
The present results suggest that there may be sex-dependent variations in the concentration of brain purines. As the purine resonance is derived primarily from adenine nucleotides, it is noteworthy that male and female sex hormones have different effects on mitochondrial function. For example, estrogens have been shown to rapidly inhibit rat brain F0F1-ATPase activity in the mitochondrial inner membrane (24) and to inhibit sodium-dependent calcium efflux from mitochondria (25). Also, estrogen has been shown to stimulate the expression of adenine nucleotide translocator mRNA in the hearts of female rats (26). Starkov and colleagues (27) reported that male sex hormones and progesterone both recouple mitochondrial energy metabolism. Thus, there are a number of mechanisms by which mitochondrial function could vary in men and women.
The limited available in vivo evidence also suggests that there are sex differences in brain energy metabolism. Riehemann and colleagues (28) have used 31P MRS to document lower values of frontal lobe phosphocreatine in women than in men. Brain phosphocreatine and ATP concentrations are maintained in equilibrium through the activity of creatine kinase. Recently we have observed (29) that the 1H MRS choline resonance is higher than normal in the caudate nucleus of depressed women but not men. Our working hypothesis is that low basal ganglia ATP is associated with a decrease in phospholipid synthesis and an accumulation of phospholipid precursors such as phosphocholine. Phosphocholine and glycerophosphoryl choline make the major contributions to the 1H MRS choline resonance (20). Phosphocholine also contributes to the 31P MRS phosphomonoester resonance. In this regard, it is notable that in depressed adults, low basal ganglia (3) and frontal lobe (4) NTP levels are associated with high phosphomonoester concentrations.
To the extent that regional abnormalities in cerebral adenosine metabolism are associated with major depression, compounds that modify adenosine metabolism may provide important new therapeutic strategies for depression. For example, S-adenosylmethionine has been assessed as a possible treatment for depression (30). The putative mechanism of action for S-adenosylmethionine is its function as a methyl donor for a range of biochemical reactions. S-Adenosylmethionine is produced by the combination of a methionine molecule and an adenosine molecule, supplied by ATP (31). Therefore, an alternative interpretation suggests that S-adenosylmethionine may have antidepressant efficacy due to its ability to increase the brain concentration of adenosine metabolites. Extending this further, the present results suggest that MRS measures of brain adenosine might be of value in predicting which depressed patients may respond to treatment with S-adenosylmethionine.
In terms of depression severity in these data, larger decrements in the proton MRS purine resonance were associated with milder episodes of depression. As milder depressive episodes are often more treatment responsive than severe episodes (32), this finding is consistent with the hypothesis that depressed subjects with low brain adenosine levels have a more positive prognosis.
Fluoxetine, the antidepressant agent used in this study, is a serotonin-specific reuptake inhibitor (33) that may interact with adenosine. In rats, adenosine has been shown to modulate serotonin release and reuptake in the hippocampus through differential effects on hippocampal A1, A2, and A3 receptors (34). Adenosine diphosphate (ADP) is produced when ATP is used as a source of high-energy phosphate in numerous biochemical processes. Intriguingly, ADP has been shown to decrease serotonin uptake into human platelets, human platelet vesicles, and rat brain synaptic vesicles (35). The present results suggest that fluoxetine’s inhibition of serotonin reuptake may be of more clinical benefit in depressed individuals with the relatively low basal ganglia ATP levels. As the 1H MRS purine resonance and the total 31P NTP resonances are lower than normal in fluoxetine responders, it is likely that cerebral ADP levels are also low. This would provide a mechanism by which fluoxetine might have different effects in responders and nonresponders. Finally, Duman (36) has emphasized that chronic administration of serotonin selective reuptake inhibitors, as well as other antidepressants, up-regulates the cyclic adenosine monophosphate (cAMP) second-messenger system. ATP is necessary for the production of cAMP, which provides yet another possible linkage between the effects of antidepressants and cerebral adenosine metabolism.
The integrated (7.5–8.5 ppm) resonance is composed of signals from a number of compounds. Van Zijl and Moonen (15) have suggested that adenosine, inosine, hypoxanthine, and xanthine may all contribute to this resonance. However, by mass, ATP is expected to contribute at least 60% of the total purine resonance intensity. Further confirming that purine 1H measures reflect mainly ATP, the intensity of the purine resonance and the beta-NTP resonance were correlated in the depressed subjects.
Hetherington and colleagues (37) recently used 31P MRS data obtained at 4 T to conclude that brain ATP concentrations in cerebral gray and white matter are 2.19 mM and 3.41 mM, respectively. These results are in excellent agreement with our observations that, in healthy volunteers, the purine MRS resonance was strongly associated with voxel white matter content. The fact that this correlation was not observed in depressed subjects presumably reflects disease-related alterations in brain purine metabolism.
In summary, we have measured the intensity of the proton MRS purine resonance in 8-cm3 basal ganglia voxels of a cohort of depressed and comparison subjects. Female depressed subjects who responded to an 8-week of course of fluoxetine, with consequent lowered Hamilton depression scale scores, were found to have lower than normal baseline purine resonance. Similarly, beta-NTP levels measured across a 45-cm3 voxel centered on the basal ganglia were also considerably lower in depressed subjects, especially responders, than in comparison subjects. These results suggest that MRS assessments of cerebral adenosine stores deserve further research and may be of value in identifying novel treatment strategies for depression and, if these novel treatments prove useful, in optimizing antidepressant efficacy.
 |
 |
Presented in part at the 8th Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine, Denver, April 1–7, 2000. Received June 12, 2000; revisions received April 18 and July 30, 2001; accepted Aug. 8, 2001. From the Brain Imaging Center, McLean Hospital. Address reprint requests to Dr. Renshaw, Brain Imaging Center, McLean Hospital, 115 Mill St., Belmont, MA 02478; [email protected] (e-mail). Supported in part by grant MH-58681 from NIMH, by an Independent Investigator Award from the National Alliance for Research on Schizophrenia and Depression, and by the Stanley Foundation Bipolar Disorders Research Center at McLean Hospital. The authors thank Peter van Zijl, Ph.D., for consultation regarding purine resonance and Ms. Anne Smith and Ms. Eileen Connelly for technical expertise in acquiring the proton MRS data.
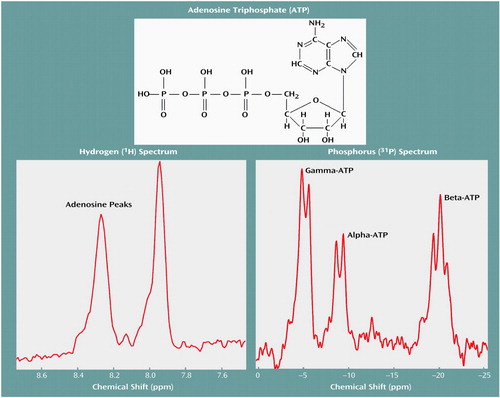
Figure 1. Magnetic Resonance Spectra Obtained From a Solution Containing 20 mM ATP at pH=7.0a
aSpectra were obtained from 250 ml of solution without spatial localization. A total of 128 transients were averaged by using a 90° pulse and TR=3 seconds.
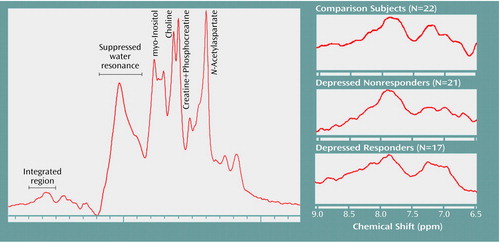
Figure 2. Summed Proton Magnetic Resonance Spectra of the Low-Field Purine Resonance in Healthy Comparison Subjects and Depressed Patients Who Did or Did Not Respond to Fluoxetine
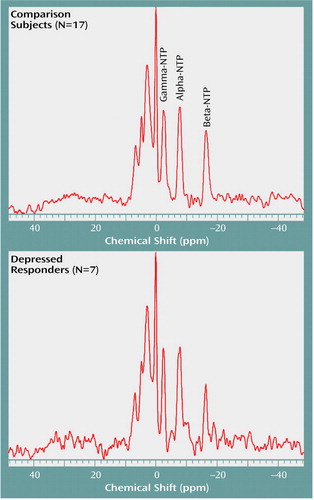
Figure 3. Nucleoside Triphosphate (NTP) Resonances as Demonstrated by Summed 31P Spectra for Healthy Comparison Subjects and Depressed Patients Who Responded to Fluoxetinea
aDepressed responders had a significantly lower beta-NTP resonance (F=26.55, df=1, 22, p<0.001).
1. Drevets WC: Functional neuroimaging studies of depression: the anatomy of melancholia. Ann Rev Med 1998; 49:341-361Crossref, Medline, Google Scholar
2. Kennedy S, Javanmard M, Vaccarino F: A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Can J Psychiatry 1997; 42:467-475Crossref, Medline, Google Scholar
3. Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF: Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry 1997; 154:116-118Link, Google Scholar
4. Volz HP, Rzanny R, Riehemann S, May S, Hegwald H, Preussler B, Hubner G, Kaiser WA, Sauer H: 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci 1998; 248:289-295Crossref, Medline, Google Scholar
5. Pissarek M, Garcia de Arriba S, Schafer M, Sieler D, Nieber K, Illes P: Changes by short-term hypoxia in the membrane properties of pyramidal cells and the levels of purine and pyrimidine nucleotides in slices of rat neocortex: effects of agonists and antagonists of ATP-dependent potassium channels. Naunyn Schmiedebergs Arch Pharmacol 1998; 358:430-439Crossref, Medline, Google Scholar
6. Younkin D: Magnetic resonance spectroscopy in hypoxia-ischemia encephalopathy. Clin Invest Med 1993; 16:115-121Medline, Google Scholar
7. Christensen JD, Kaufman MJ, Levin JM, Mendelson JH, Holman BL, Cohen BM, Renshaw PF: Abnormal cerebral metabolism in polydrug abusers during early withdrawal: a 31P MR spectroscopy study. Magn Reson Med 1996; 35:658-663Crossref, Medline, Google Scholar
8. Deicken RF, Calabrese G, Merrin EL, Fein G, Weiner MW: Basal ganglia phosphorous metabolism in chronic schizophrenia. Am J Psychiatry 1995; 152:126-129Link, Google Scholar
9. Buchsbaum M, Hazlett E: Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr Bull 1998; 24:343-364Crossref, Medline, Google Scholar
10. Volkow N, Wang G, Fowler J: Imaging studies of cocaine in the human brain and studies of the cocaine addict. Ann NY Acad Sci 1997; 820:41-54Crossref, Medline, Google Scholar
11. Moore C, Renshaw P: Magnetic resonance spectroscopy studies of affective disorders, in Brain Imaging in Clinical Psychiatry. Edited by Krishna K, Doraiswamy P. New York, Marcel Dekker, 1997, pp 185-214Google Scholar
12. Rauch SL, Renshaw PF: Clinical neuroimaging in psychiatry. Harv Rev Psychiatry 1995; 2:297-312Crossref, Medline, Google Scholar
13. Decanniere C, Eleff S, Davis D, van Zijl PC: Correlation of rapid changes in the average water diffusion constant and the concentrations of lactate and ATP breakdown products during global ischemia in cat brain. Magn Reson Med 1995; 34:343-352Crossref, Medline, Google Scholar
14. Mori S, Eleff SM, Pilatus U, Mori N, van Zijl PC: Proton NMR spectroscopy of solvent-saturable resonances: a new approach to study pH effects in situ. Magn Reson Med 1998; 40:36-42Crossref, Medline, Google Scholar
15. van Zijl PC, Moonen CT: In situ changes in purine nucleotide and N-acetyl concentrations upon inducing global ischemia in cat brain. Magn Reson Med 1993; 29:381-385Crossref, Medline, Google Scholar
16. Renshaw PF, Lafer B, Babb SM, Fava M, Stoll AL, Christensen JD, Moore CM, Yurgelun-Todd DA, Bonello CM, Pillay SS, Rothschild AJ, Nierenberg AA, Rosenbaum JF, Cohen BM: Basal ganglia choline levels in depression and response to fluoxetine treatment: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry 1997; 41:837-843Crossref, Medline, Google Scholar
17. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
18. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
19. Fleiss J, Cohen J, Everitt B: Large sample standard errors of kappa and weighted kappa. Psychol Bull 1969; 72:323-327Crossref, Google Scholar
20. Miller B, Chang L, Booth R, Ernst T, Cornford M, Nikas D, McBride D, Jenden D: In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci 1996; 58:1929-1935Crossref, Medline, Google Scholar
21. Miller R: Simultaneous Statistical Analysis. New York, John Wiley & Sons, 1981Google Scholar
22. Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S: Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord 2000; 60:13-23Crossref, Medline, Google Scholar
23. Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J: Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry 2000; 57:285-290Crossref, Medline, Google Scholar
24. Zheng J, Ramirez VD: Rapid inhibition of rat brain mitochondrial proton F0F1-ATPase activity by estrogens: comparison with Na+, K+ -ATPase of porcine cortex. Eur J Pharmacol 1999; 368:95-102Crossref, Medline, Google Scholar
25. Horvat A, Petrovic S, Nedeljkovic N, Martinovic JV, Nikezic G: Estradiol affect Na-dependent Ca2+ efflux from synaptosomal mitochondria. Gen Physiol Biophys 2000; 19:59-71Medline, Google Scholar
26. Too CK, Giles A, Wilkinson M: Estrogen stimulates expression of adenine nucleotide translocator ANT1 messenger RNA in female rat hearts. Mol Cell Endocrinol 1999; 150:161-167Crossref, Medline, Google Scholar
27. Starkov AA, Simonyan RA, Dedukhova VI, Mansurova SE, Palamarchuk LA, Skulachev VP: Regulation of the energy coupling in mitochondria by some steroid and thyroid hormones. Biochim Biophys Acta 1997; 1318:173-183Crossref, Medline, Google Scholar
28. Riehemann S, Volz HP, Wenda B, Hubner G, Rossger G, Rzanny R, Sauer H: Frontal lobe in vivo (31)P-MRS reveals gender differences in healthy controls, not in schizophrenics. NMR Biomed 1999; 12:483-489Crossref, Medline, Google Scholar
29. Sonawalla SB, Moore CM, Renshaw PF, Graham LE, Vega NA, Fava M, Lafer B, Nierenberg A: Proton magnetic resonance spectroscopy study of treatment-resistant depression, in 2000 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 2000, p 64Google Scholar
30. Fava M, Giannelli A, Rapisarda V, Patralia A, Guaraldi GP: Rapidity of onset of the antidepressant effect of parenteral S-adenosyl-l-methionine. Psychiatry Res 1995; 56:295-297Crossref, Medline, Google Scholar
31. Baldessarini RJ: Neuropharmacology of S-adenosyl-l-methionine. Am J Med 1987; 83(5A):95-103Google Scholar
32. Schatzberg AF: Antidepressant effectiveness in severe depression and melancholia. J Clin Psychiatry 1999; 60(suppl 4):14-21Google Scholar
33. Masand PS, Gupta S: Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry 1999; 7:69-84Crossref, Medline, Google Scholar
34. Okada M, Kawata Y, Murakami T, Wada K, Mizuno K, Kondo T, Kaneko S: Differential effects of adenosine receptor subtypes on release and reuptake of hippocampal serotonin. Eur J Neurosci 1999; 11:1-9Crossref, Medline, Google Scholar
35. Anderson G, Hall L, Horne W, Yang J: Adenosine diphosphate inhibits the serotonin transporter. Biochim Biophys Acta 1996; 1283:14-20Crossref, Medline, Google Scholar
36. Duman R: Novel therapeutic approaches beyond the serotonin receptor. Biol Psychiatry 1998; 44:324-335Crossref, Medline, Google Scholar
37. Hetherington HP, Spencer DD, Vaughan JT, Pan JW: Quantitative (31)P spectroscopic imaging of human brain at 4 Tesla: assessment of gray and white matter differences of phosphocreatine and ATP. Magn Reson Med 2001; 45:46-52Crossref, Medline, Google Scholar



