Enhancing Comprehension of Consent for Research in Older Patients With Psychosis: A Randomized Study of a Novel Consent Procedure
Abstract
OBJECTIVE: The ability of individuals with schizophrenia to provide informed consent for research has become the focus of public debate. The authors examined whether a novel consent procedure improved the comprehension of consent for older patients with psychosis. METHOD: Fifty outpatients with schizophrenia or other psychotic disorders and 19 normal comparison subjects aged 40–80 were randomly assigned to groups given either a routine or an enhanced consent procedure. The latter utilized a computerized presentation that included sequential bullet points and summaries of key information. A posttest measured comprehension of consent-relevant information. RESULTS: A significantly greater proportion of patients who received the enhanced consent procedure scored 100% on first and second trials of the posttest, compared to those receiving the routine procedure (trial 1: 42.3% versus 8.3%; trial 2: 80.8% versus 45.8%, respectively). CONCLUSIONS: The enhanced consent method improved comprehension of information relevant for consent in older patients with psychosis.
A relatively recent National Bioethics Advisory Commission report (1) focused on psychiatric patients’ heightened vulnerability to impaired decisional capacity (2, 3). Existing research is inconsistent, however, regarding whether psychiatrically ill individuals are more prone to poor comprehension of consent-relevant information. In a series of studies of treatment-related consent (4), persons with schizophrenia were more likely to demonstrate deficits on measures of decisional capacity compared to medically ill patients and community comparison subjects; however, the schizophrenia patients also showed wider variability in performance. Furthermore, the majority of schizophrenia patients were not impaired on any given measure of capacity (4). Relatively little is known about enhancing the comprehension of consent forms. Better organization of formats, feedback from previous trials, and multiple learning trials have been shown to improve subjects’ comprehension of consent for research and treatment (5). In two studies (6, 7), persons with schizophrenia showed improved comprehension of research consent when educational strategies were employed.
We designed a novel procedure for research consent and evaluated its use in older patients with psychosis and normal comparison subjects to determine whether there were differential benefits of the enhanced consent procedure in patients with persistent mental illness. We hypothesized that 1) on each posttest trial to assess comprehension of consent, a higher cumulative proportion of subjects within each diagnostic group (patients and normal comparison subjects) who received the enhanced consent procedure would obtain 100% correct responses compared to those given the routine consent procedure and 2) patients and normal comparison subjects receiving the enhanced consent procedure would score higher on the posttest compared to those receiving the routine consent procedure.
Method
The protocol was approved by our university’s institutional review board. The study included 50 middle-aged (age 40 years or more) or elderly patients who enrolled in the Intervention Research Center for Psychosis in Older People. Subjects with dementia or recent (within 6 months) substance abuse or dependence were excluded. The majority (N=36, 72%) of the patients met DSM-IV criteria for schizophrenia (N=23, 46%) or schizoaffective disorder (N=13, 26%); others had mood disorder with psychotic features (N=13, 26%) or psychosis not otherwise specified (N=1, 2%). The mean duration of illness was 24.0 years (SD=11.2). Nineteen demographically equivalent normal comparison subjects who were free of major neuropsychiatric illness were recruited through community centers, churches, and senior living centers.
Subjects were randomly assigned to receive the routine or enhanced consent procedure. The 3.5-page consent form described research including psychiatric, medical, and psychosocial interviews, neuropsychological tests, motor assessments, blood sampling, and magnetic resonance imaging (MRI). The consent form also discussed the purpose of the Intervention Research Center for Psychosis in Older People (to study psychosis and its management in older individuals), the time required for baseline evaluations (approximately 2 days), the risks of participation (e.g., bruising or infection from blood sampling, feeling claustrophobic during MRI), the benefits of participation (to help researchers learn about psychotic disorders and treatments), the voluntary nature of participation, and whom to call with questions.
Bachelor’s- or master’s-level raters were trained to administer both versions of the consent procedures to minimize the effects of individual styles. For the routine consent procedure, the rater read the entire consent form aloud while the subject read his or her own copy. The staff member stopped occasionally to review key points. Staff were instructed to provide the routine consent procedure exactly as they had done for participants in research at the Intervention Research Center for Psychosis in Older People before this study. Staff members were observed several times in both conditions to ensure uniformity. The time needed to administer the consent procedures and the posttest averaged 30 minutes for the routine procedure and 40 minutes for the enhanced procedure.
For the enhanced procedure, the routine procedure was converted into a computerized presentation that retained all material from the written consent form. Each graphic was titled to structure the presentation, summary graphics were added (before, after, and during the presentation) to review key information, and bullet points were presented sequentially. As was done for the routine consent procedure, the presentation for the enhanced procedure was read aloud; subjects were invited to ask questions at any time.
Immediately after the consent procedure, 20 questions assessing comprehension (yes/no and short answers; maximum possible score=20) were read aloud by the examiner. Questions involved the illness being studied, procedures, potential risks and benefits, voluntariness, ability to withdraw, and confidentiality. Examples included, “If you want to, when could you quit this study?” and, “Describe two risks of participation.”
Subjects were allowed up to three trials. At trial 1, the entire test was given without the opportunity to receive feedback and without the consent form available. Feedback from trial 1 was offered between consecutive posttest trials by repeating (in the same format as originally provided) the information relevant to the incorrectly answered questions, and the number of questions answered correctly on subsequent trials was added to the previous score. At trials 2 and 3 (which took an additional 5 minutes each) the subjects could refer to the consent form.
Continuous variables were transformed to meet assumptions of normality when necessary. We used two-way analyses of variance with two grouping factors, diagnostic group (patients and normal comparison subjects) and consent procedure (routine and enhanced), to compare scores on each trial separately. Repeated measures analysis was not used because scores on trials 2 and 3 represented cumulative scores that included the total correct items from the previous trials. Scores for trial 3 were not analyzed because almost all of the subjects scored 100% by trial 3. Two-by-two chi-square analysis with Yates’s correction was used to compare the proportions of subjects (within each diagnostic group) who obtained 100% correct responses on each trial across consent conditions. All tests of significance were two-tailed with alpha set at 0.05.
Results
The patients and normal comparison subjects did not differ in terms of demographic characteristics (patients: mean age=51.3 years, SD=8.5, education=12.4 years, SD=2.9, 44% [N=22] men, 78% [N=39] Caucasian; normal comparison subjects: mean age=55.2, SD=9.6, education=13.8 years, SD=1.8, 58% [N=11] men, 68% [N=13] Caucasian). There were also no significant demographic differences within either the patient group or the normal comparison group across consent conditions (routine versus enhanced procedures).
Compared to the percentage of the 24 patients who received the routine consent procedure (N=2, 8.3%), a greater cumulative proportion of the 26 patients who received the enhanced procedure (N=11, 42.3%) scored 100% at trial 1 and at trial 2 (routine procedure: N=11, 45.8%; enhanced procedure: N=21, 80.8%) (Figure 1). The percentages of normal comparison subjects with perfect scores, however, did not differ across consent conditions (trial 1: routine procedure: 4 of 10, 40.0%; enhanced procedure: 6 of 9, 66.7%; trial 2: routine procedure: 100%; enhanced procedure: 8 of 9, 88.9%).
Mean scores on the posttest were as follows: normal comparison subjects—trial 1: routine procedure: 19.0 (SD=0.9), enhanced procedure: 19.6 (SD=0.7); trial 2: routine procedure: 20.0 (SD=0.0), enhanced procedure: 19.9 (SD=0.3); patients—trial 1: routine procedure: 15.8 (SD=2.9), enhanced procedure: 18.4 (SD=1.9); trial 2: routine procedure: 18.9 (SD=1.5), enhanced procedure, 19.7 (SD=0.7). The two-way interaction of diagnostic group and consent condition was not significant for either trial (trial 1: F=1.76, df=1, 65, p=0.19; trial 2: F=3.69, df=1, 65, p=0.06). Main effects for consent condition were significant for trial 1 (F=9.52, df=1, 65, p=0.003) but not for trial 2 (F=1.63, df=1, 65, p=0.21). Main effects for diagnostic group were significant for trial 1 (F=15.46, df=1, 65, p=0.001) and trial 2 (F=7.54, df=1, 65, p=0.008). It appeared, particularly among patients, that the enhanced consent procedure was associated with higher scores at trial 1 and, to a lesser extent, at trial 2. There was an apparent ceiling effect for the performance of the normal comparison subjects at trials 1 and 2, whereas the patients showed greater variability in scores on the posttest.
Discussion
Even after two trials, 54% and 19% of the patients receiving the routine or enhanced consent procedure, respectively, answered at least one question incorrectly. Thus it appears that persons with schizophrenia and other psychoses may be more vulnerable than normal subjects to deficits in comprehension of research consent. However, our findings also suggest that when a concerted effort is made to impart crucial consent information, even older patients with chronic psychotic disorders show improved postconsent understanding.
Our findings extend work by two other research groups (6, 7) that used somewhat different procedures in younger adults with schizophrenia. The present study differs from those studies in that we randomly assigned both patients and normal comparison subjects to routine or enhanced consent procedures. We know of no other published randomized studies of consent for research that used a computer-based enhanced presentation of the consent form. In addition, our focus was on middle-aged and older patients with schizophrenia and other psychotic disorders. The limitations of this study include a lack of follow-up testing and the small size of the group of normal subjects. Also, we did not include an assessment of comprehension with a standardized instrument, such as the MacArthur Competence Assessment Tool—Clinical Research (8).
Future studies should follow subjects longitudinally, since disease fluctuations may affect decision-making capacity. Interventions employing enhanced educational strategies—e.g., feedback from previous trials, repeated learning trials, and use of programmed learning technologies—warrant exploration (9).
Received Sept. 26, 2000; revisions received Feb. 16 and April 17, 2001; accepted May 7, 2001. From the Department of Psychiatry, University of California, San Diego; the Psychiatry Service, VA San Diego Healthcare System; and the Department of Family and Preventive Medicine and the Department of Medicine, University of California, San Diego. Address reprint requests to Dr. Dunn, Geriatric Psychiatry Division 116A-1, VA San Diego Healthcare System, 3350 La Jolla Village Dr., San Diego, CA 92161; [email protected] (e-mail). Supported in part by NIMH grants MH-43693, MH-51459, MH-45131, and MH-49671 and by the Department of Veterans Affairs.
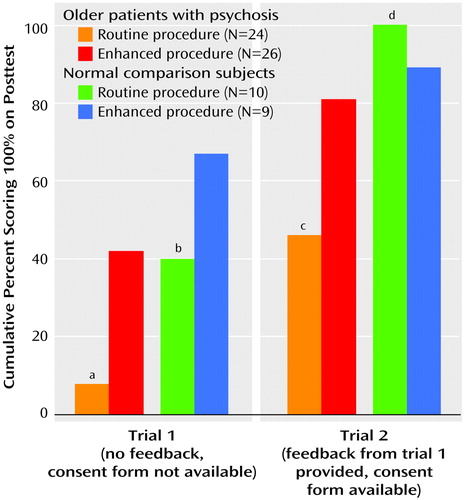
Figure 1. Rates of Perfect Scores on Assessment of Comprehension for Subjects Who Were Randomly Assigned to be Given Either a Routine or Enhanced Procedure for Consent
aSignificant difference between patients receiving routine and enhanced procedures (χ2=5.82, df=1, p=0.02, with Yates’s correction).
bNonsignificant difference between comparison subjects receiving routine and enhanced procedures (χ2=0.49, df=1, p=0.50, with Yates’s correction).
cSignificant difference between patients receiving routine and enhanced procedures (χ2=5.18, df=1, p=0.03, with Yates’s correction).
dNonsignificant difference between comparison subjects receiving routine and enhanced procedures (χ2=0.00, df=1, p=0.96, with Yates’s correction).
1. Research Involving Persons With Mental Disorders That May Affect Decision-Making Capacity: Report of the Recommendations of the National Bioethics Advisory Commission. Rockville, Md, National Bioethics Advisory Commission, 1998Google Scholar
2. Michels R: Are research ethics bad for our mental health? N Engl J Med 1999; 340:1427-1430Crossref, Medline, Google Scholar
3. Oldham JM, Haimowitz S, Delano SJ: Protection of persons with mental disorders from research risk: a response to the report of the National Bioethics Advisory Commission. Arch Gen Psychiatry 1999; 56:688-693Crossref, Medline, Google Scholar
4. Grisso T, Appelbaum PS: The MacArthur Treatment Competence Study, III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav 1995; 19:149-174Crossref, Medline, Google Scholar
5. Dunn LB, Jeste DV: Enhancing informed consent for research and treatment. Neuropsychopharmacology 2001; 24:595-607Crossref, Medline, Google Scholar
6. Carpenter WT, Gold JM, Lahti AC, Queern CA, Conley RR, Bartko JJ, Kovnick J, Appelbaum PS: Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry 2000; 57:533-538Crossref, Medline, Google Scholar
7. Wirshing DA, Wirshing WC, Marder SR, Liberman RP, Mintz J: Informed consent: assessment of comprehension. Am J Psychiatry 1998; 155:1508-1511Link, Google Scholar
8. Appelbaum PS, Grisso T: The MacArthur Competence Assessment Tool—Clinical Research. Sarasota, Fla, Professional Resources Press, 1996Google Scholar
9. Rosoff AJ: Informed consent in the electronic age. Am J Law Med 1999; 25:367-386Medline, Google Scholar



