Cost-Effectiveness of a Collaborative Care Program for Primary Care Patients With Persistent Depression
Abstract
OBJECTIVE: The authors evaluated the incremental cost-effectiveness of stepped collaborative care for patients with persistent depressive symptoms after usual primary care management. METHOD: Primary care patients initiating antidepressant treatment completed a standardized telephone assessment 6–8 weeks after the initial prescription. Those with persistent major depression or significant subthreshold depressive symptoms were randomly assigned to continued usual care or collaborative care. The collaborative care included systematic patient education, an initial visit with a consulting psychiatrist, 2–4 months of shared care by the psychiatrist and primary care physician, and monitoring of follow-up visits and adherence to medication regimen. Clinical outcomes were assessed through blinded telephone assessments at 1, 3, and 6 months. Health services utilization and costs were assessed through health plan claims and accounting data. RESULTS: Patients receiving collaborative care experienced a mean of 16.7 additional depression-free days over 6 months. The mean incremental cost of depression treatment in this program was $357. The additional cost was attributable to greater expenditures for antidepressant prescriptions and outpatient visits. No offsetting decrease in use of other health services was observed. The incremental cost-effectiveness was $21.44 per depression-free day. CONCLUSIONS: A stepped collaborative care program for depressed primary care patients led to substantial increases in treatment effectiveness and moderate increases in costs. These findings are consistent with those of other randomized trials. Improving outcomes of depression treatment in primary care requires investment of additional resources, but the return on this investment is comparable to that of many other widely accepted medical interventions.
Improving management of depression in primary care is a public health priority. Depression is one of the most common major health conditions managed in primary care (1, 2). The impact of depression on functioning and quality of life equals or exceeds that of most other chronic health conditions (3–5). Abundant evidence demonstrates that current management of depression falls far short of that recommended by evidence-based guidelines (6–8). Several randomized trials have demonstrated that organized treatment programs for depressed primary care patients can significantly improve quality of treatment and clinical outcomes. Schulberg and colleagues (9) reported that guideline-based depression treatment (either pharmacotherapy or psychotherapy) was superior to usual primary care in both clinical and functional outcomes. Katzelnick and colleagues (10) reported similar benefits from a systematic depression management program for patients who used high levels of general medical care. We have previously described the clinical benefits of three different collaborative care models for depressed primary care patients (11–13) as well as benefits of a less intensive telephone care management program (14). Wells and colleagues (15) have documented significant benefits when guideline-based depression treatment programs are disseminated across a wide range of primary care settings.
Widespread adoption of these systematic treatment programs will depend on the balance of incremental benefits and incremental cost. Data on cost-effectiveness have been published for several of the effective treatment programs just described. Lave et al. (16) found that the mean incremental outpatient health services costs over 1 year were approximately $740 for guideline-based pharmacotherapy and $840 for interpersonal psychotherapy. The depression management program tested by Katzelnick and colleagues for patients with high utilization levels (10, 17) led to a mean increase of approximately $675 in outpatient costs to participating health plans over 12 months. We previously reported (18) that the mean 1-year incremental outpatient costs were $487 for a psychiatrist collaborative care program and $264 for a psychologist collaborative care program.
In this report we describe the incremental cost and cost-effectiveness of the third type of collaborative care for depression we have studied (13). In this stepped collaborative care program, the intervention was reserved for patients with persistent depressive symptoms after 6–8 weeks of treatment by primary care physicians. We hoped that this stepped-care approach would result in more efficient use of psychiatric consultation resources. As reported previously (13), this program resulted in significantly better antidepressant treatment and clinical outcomes than did usual primary care.
Method
Participants were recruited from three large primary care clinics of Group Health Cooperative. Group Health Cooperative is a group-model health maintenance organization serving approximately 400,000 members in Washington state.
Computerized pharmacy and visit registration records were used to identify all patients receiving a new antidepressant prescription from a primary care physician associated with a visit diagnosis of depressive disorder (including physician diagnoses of major depressive episode, dysthymia, and depressive disorder not otherwise specified). A “new” prescription was defined by an interval of at least 120 days since last use of an antidepressant (based on expected expiration date of most recent antidepressant prescription in computerized pharmacy records). An invitation letter was mailed to each potentially eligible patient approximately 1 month after the initial prescription. Potential participants were then contacted by telephone 6–8 weeks after the initial prescription. After completing a documented oral consent procedure, participants completed a telephone screening assessment that included the current and past depression modules of the Structured Clinical Interview for DSM-IV (SCID) (19). Patients were invited to participate in an in-person baseline assessment on the basis of the following criteria: significant residual symptoms (i.e., four or more of the nine DSM-IV depression criteria), history of dysthymia (i.e., depressive symptoms present for 2 years or more), or history of recurrent depression (i.e., two or more prior depressive episodes). The in-person baseline assessment included a 20-item depression scale extracted from the SCL-90 (20) as well as several other measures not presented here. Final eligibility was based on both the SCID and SCL-90 results. A patient with significant residual symptoms according to the SCID (i.e., at least four of the nine DSM-IV depression criteria) was considered eligible if the SCL-90 depression score was 1.00 or higher. Other patients were also enrolled if their SCL-90 depression scores were 1.50 or higher. The criteria for exclusion were as follows: score of 2 or higher on the CAGE alcohol questionnaire, plans to disenroll from Group Health Cooperative within 12 months, recent use of mood stabilizer or antipsychotic medication, pregnancy or breast-feeding, and current medication management by a psychiatrist. After a full explanation of the study procedures, written informed consent was obtained from all participants.
The patients assigned to the group receiving usual care could obtain any services normally available inside or outside of Group Health Cooperative—including referral to specialty mental health care. No additional services were provided, but no services usually available were limited or withheld.
The collaborative care program was a multifaceted intervention including the following components:
1. An educational book and videotape regarding effective management of depression.
2. Two to four consultation visits with a liaison psychiatrist practicing in the primary care clinic.
3. Algorithm-based adjustment of antidepressant pharmacotherapy.
4. As-needed referral to psychosocial treatment or community resources.
5. Ongoing monitoring of adherence to medication regimen.
During this period of collaborative care, most patients alternated follow-up visits with the liaison psychiatrist and primary care physician. After 3–4 months, responsibility for ongoing depression care was transferred back to the primary care physician (with specialty mental health services available as in usual care). Liaison psychiatrists continued to monitor treatment adherence and provide as-needed consultation to the primary care physicians throughout the follow-up period.
Clinical outcomes were assessed through blinded telephone interviews 1, 3, and 6 months after randomization. These interviews included repeat administration of the SCL-90 depression scale (the primary outcome measure), questions regarding visits to health care providers outside Group Health Cooperative, and other measures not presented here.
Computerized health plan data were used to identify all health services provided or paid for by Group Health Cooperative during the 6 months after randomization (inpatient and outpatient services for mental health or general medical care). All outpatient and inpatient services provided by Group Health Cooperative were assigned costs based on health plan accounting records (including actual personnel, supply, and overhead costs). Services purchased by Group Health Cooperative from external providers were assigned costs equal to the amount reimbursed by Group Health Cooperative. Visits to the consulting psychiatrist were assigned costs of $90 for each 50-minute visit and $55 for each 25-minute visit. Computerized pharmacy data were used to calculate the Chronic Disease Score (21, 22), a measure of chronic medical comorbidity.
Following the method of Lave et al. (16), we used SCL-90 depression scores from the baseline and follow-up assessments to calculate the number of depression-free days during the 6-month follow-up period. This method uses depression severity data from two consecutive outcome assessments to estimate depression severity for each day during the interval (by linear interpolation). Days with an SCL-90 depression score of 0.50 or less are considered “depression free.” On days with an SCL-90 depression score of 2.00 or higher the patient is considered “fully symptomatic.” Days with intermediate severity scores are assigned a value between depression free and fully symptomatic by linear interpolation (e.g., days with an SCL-90 score of 1.25 would be considered 50% depression free). Values for each follow-up interval are then summed to yield the total number of depression-free days during the follow-up period. We also conducted sensitivity analyses using varying thresholds for “depression free” (SCL-90 score ranging from 0.25 to 0.75) and “fully symptomatic” (SCL-90 score ranging from 1.50 to 2.50). These alternatives yielded different values for total number of depression-free days but had minimal impact (i.e., less than 10% change) on the difference in depression-free days between the collaborative care and usual care groups. Calculation of depression-free days was limited to participants completing all follow-up assessments.
In our primary analyses of cost data we defined “depression treatment costs” as the estimated costs for all antidepressant prescriptions, all outpatient specialty mental health care, all visits to the collaborative care psychiatrist (for patients in the intervention group), and all primary care visits associated with a mental health diagnosis. In the secondary analyses we considered the broader category of total outpatient health services costs. We also examined differences in total health services costs, but the number of participants did not allow sufficient statistical power to detect even moderate differences in total costs. Because we lacked the detail necessary to estimate the costs of out-of-plan visits not paid for by Group Health Cooperative, those visits were not included in the cost calculations.
We estimated confidence intervals (CIs) for both depression-free days and cost measures by bootstrap resampling with 1,000 draws using bias correction (23). Standard bootstrap approaches were possible because both depression-free days and costs are single-number summaries of longitudinal outcomes. Adjusted differences between the groups receiving collaborative care and usual care were estimated by using regression models with bootstrap interval estimates. The bootstrap analyses were carried out by using the Stata statistical software package (24). All models included adjustment for age, sex, baseline SCL-90 depression score, and Chronic Disease Score.
Results
A total of 228 patients were enrolled in the randomized trial. Their mean age was 47 years (SD=14), and 74% were female (N=169). The mean baseline SCL-90 depression score was 1.92 (SD=0.51), indicating moderate depression. Approximately 80% of the patients (N=182) reported a history of recurrent depression (at least two previous episodes), and 55% (N=125) reported a history of dysthymia. The patients assigned to collaborative care (N=114) and usual care (N=114) differed significantly in the proportion of women: 67% of the patients in collaborative care (N=76) and 82% of the patients receiving usual care (N=93) (χ2=5.2, df=1, p=0.02). The two groups did not differ in age, education, employment, ethnicity, baseline depression severity, or medical comorbidity (13).
All three blinded outcome assessments were completed by 76% of the patients receiving collaborative care (N=87) and 71% of those receiving usual care (N=81). Follow-up participation was not significantly related to any clinical or demographic characteristic assessed at baseline. The proportion of patients enrolled in the health plan for at least 150 days of the 180-day follow-up period was 96% for both collaborative care (N=110) and usual care (N=109). Cost comparisons were limited to patients enrolled throughout the follow-up. Comparisons of the measures of effectiveness (depression-free days) and cost-effectiveness were limited to patients completing all follow-up assessments.
Figure 1 displays the proportion of depression-free days over the 6 months of follow-up. The total numbers of depression-free days during follow-up for the collaborative care and usual care groups were calculated as the areas under the curves shown in Figure 1. By this measure, the mean number of depression-free days was 87.7 (95% CI=76.6–96.7) for the collaborative care group and 70.9 (95% CI=60.8–81.3) for the usual care group. After adjustment for patient age, sex, baseline SCL-90 depression score, and Chronic Disease Score, the incremental number of depression-free days attributable to the collaborative care intervention was significantly greater than zero (t=2.28, df=166, p=0.02, adjusted difference=16.7, 95% CI for difference=1.3–31.0). While the difference between groups in mean SCL-90 depression scores decreased between the 3- and 6-month assessments, the difference in cumulative depression-free days (i.e., difference between areas under the two curves) continued to increase.
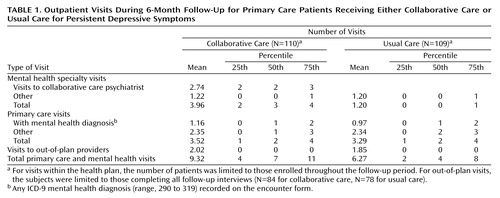
Table 1 displays utilization of outpatient services for the two groups over 6 months. As reported previously (13), patients in the intervention group made a mean of 2.74 visits to the collaborative care psychiatrist. In all other categories, the mean numbers of visits were similar in the collaborative care and usual care groups.
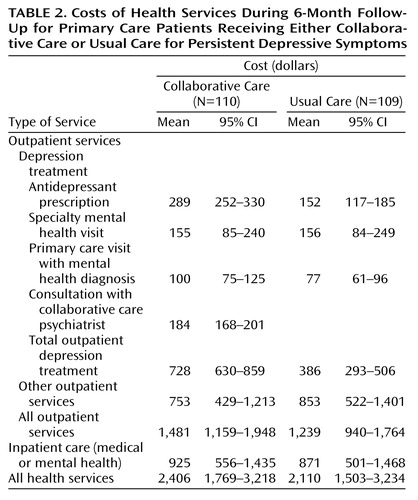
Table 2 displays estimated costs in various categories over 6 months. Consistent with the goals of the intervention program, the additional costs for collaborative care patients were concentrated in two categories: antidepressant prescriptions and outpatient visits. The depression treatment costs were approximately $340 greater for the collaborative care group. Similar differences were seen for broader categories of health services costs (approximately $240 for total outpatient services, $300 for total health services). In other words, the estimated costs for general medical services (services not directed at depression or other mental health treatment) were similar in the collaborative care and usual care groups. As indicated by the confidence intervals, the precision of these estimates was relatively good for depression treatment costs and progressively poorer for broader cost measures.
Table 3 displays the adjusted incremental cost and the adjusted incremental cost-effectiveness ratio for three categories of health services. For depression treatment cost (our primary outcome measure), the 95% CI for incremental cost-effectiveness ranged from approximately $8 per depression-free day (reflecting the lower bound of incremental cost and upper bound of incremental effectiveness) to approximately $125 (reflecting the lower bound of incremental effectiveness and upper bound of incremental cost). As expected, the 95% CIs increased progressively with broader categories of cost. For both total outpatient costs and total health services costs, we cannot exclude a finding of cost offset (negative incremental cost), but we also cannot exclude cost-effectiveness ratios of $200–$300 per depression-free day.
Our final analyses examined the likelihood that either collaborative care or usual care would show both greater effectiveness and lower cost (a “dominant” alternative in the jargon of cost-effectiveness). In 1,000 bootstrap replications, usual care was found to have both greater effectiveness and lower cost only 16 times, indicating a less than 2% probability that usual care would dominate over collaborative care. Dominance of the collaborative care intervention (i.e., greater effectiveness and lower cost) was not observed in 1,000 replications. In other words, we found a greater than 98% probability that collaborative care would lead to both increased cost and increased effectiveness.
Discussion
In this group of patients with persistent symptoms after initial primary care treatment, a collaborative care program led to both increases in the amount of time without depression and increases in health services costs. This finding places our collaborative care intervention in the same category as most other clinical interventions in mental health or general medical care in that achieving better clinical outcomes required additional expenditures.
Several points should be considered in interpreting our findings. First, we did not consider the effect of depression or depression treatment from broader perspectives, such as that of the employer (e.g., lost productivity) or the larger society (e.g., effects on educational attainment [25], earning potential [26], or marital stability [27]). Second, our analyses were limited to 6 months (the period for which clinical effectiveness data were available). Were we able to assess effectiveness and cost over a longer period, it is likely that incremental benefits would continue to accumulate while costs of maintenance treatment would be lower than those during the acute phase. Third, our estimates of additional medication costs (the largest component of additional costs) did not include the expected lower cost of generic antidepressant drugs. Finally, our calculation of depression-free days was based on the SCL-90 depression scale rather than the Hamilton Depression Rating Scale used in previous studies.
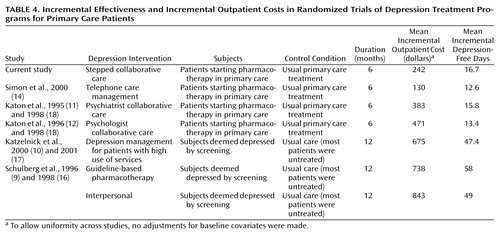
These findings are broadly consistent with those reported from several other randomized trials. Table 4 summarizes data on incremental effectiveness and incremental costs from this study and five other randomized trials of organized depression treatment programs: our study of telephone care management for primary care patients treated with antidepressants (14), our two previous studies of collaborative care for depression (11, 12, 18), the study by Katzelnick et al. (10, 17) of a depression management program for patients who used high levels of medical care, and the study by Schulberg et al. (9, 16) of guideline-based psychotherapy and pharmacotherapy. The various programs differed widely in both incremental effectiveness and incremental costs. Some of this variation reflects differences in the intensity of the interventions tested (e.g., 16 psychotherapy sessions in the interpersonal psychotherapy program reported by Schulberg et al. versus two telephone contacts in our telephone care management study). Some of the variation also reflects differences in the length of follow-up (12 months in the Schulberg et al. and Katzelnick et al. studies versus 6 months in all others) and differences in control or comparison groups (usual care in our previous studies versus largely untreated control groups in the Schulberg et al. and Katzelnick et al. studies). Longer follow-up and comparison to a largely untreated control group would be expected to increase both incremental cost and incremental depression-free days. Despite this variation, the results of these seven randomized trials suggest a clear pattern—incremental benefit is roughly proportional to the additional resources invested in depression care.
The stepped-care approach used in this study does differ in philosophy from the more general collaborative care approaches used in previous intervention studies by our group (11, 12). This intervention was limited to the 30%–40% of patients with unsatisfactory outcomes after initial primary care management rather than the broader population of all primary care patients beginning depression treatment. While the per-patient costs may be similar, the total cost of a population-based targeted or stepped-care approach should be considerably less. The degrees of efficiency (measured as dollars per additional depression-free day) may be similar for diffuse and targeted approaches, but a targeted approach may be preferable when the available resources (e.g., psychiatric personnel) are fixed. This study and our recent study of brief telephone contacts for medication monitoring and care management (14) could be viewed as two components of a comprehensive population-based approach to depression treatment. Telephone care management is a relatively inexpensive approach suitable for the large population of patients initiating depression treatment. Stepped collaborative care is a more intensive and expensive approach suitable for patients with persistent symptoms after initial primary care treatment.
The incremental costs of our intervention program concentrated in the areas one would expect given the goals of the intervention program. The collaborative care patients made approximately three additional follow-up visits and were also more likely to continue taking antidepressant medication. Consistent with these effects of the intervention program, the cost of visits was approximately $200 higher and the cost of antidepressant prescriptions was approximately $150 higher in the collaborative care group.
Our data are not consistent with the hypothesis that improved depression treatment reduces overall health services costs. The depression treatment costs were approximately $340 higher in the collaborative care group, and we did not observe decreases in other categories of cost sufficient to offset the increased expenditures for antidepressant prescriptions and follow-up visits. While our estimates of “nondepression” health services costs are subject to considerable error, our best estimate is that these other components of cost did not differ significantly between the collaborative care and usual care patients. This finding is also broadly consistent with those from other randomized trials of depression care programs (10, 16, 18). Each of the other studies listed in Table 4 showed a significant increase in expenditures for depression treatment as well as a similar increase in overall outpatient expenditures. In the more intensive programs (our two previous collaborative care studies [11, 12, 18], the Katzelnick et al. depression management program [10, 17], and the two interventions in the Schulberg et al. study [9, 16]) the increased number of outpatient visits required for depression treatment was somewhat offset by a reduction in other outpatient utilization (i.e., a partial cost-offset effect).
At the level of management and policy, the need to improve depression care must compete with other priorities. The decision to invest additional resources in improved depression treatment or other health care interventions will depend on the value created by these different investments—with value expressed as incremental cost per unit of incremental benefit. Comparing benefit or effectiveness across health care interventions requires a common measure such as health utility, commonly expressed as quality-adjusted life years. While several methods have been proposed for translating the impact of depression into quality-adjusted life years, no single method is yet well established or widely accepted. Our review of available evidence (28–33) suggests that a transition from fully symptomatic depression to full remission is associated with an improvement in health utility between 0.2 and 0.4 (i.e., an increase of 0.2 to 0.4 quality-adjusted life years per year). Based on this range for conversion, our estimate of $21.44 per additional depression-free day would be equal to an estimate of $19,564 to $39,128 per quality-adjusted life year gained.
Our findings suggest that additional spending on improved depression treatment is a prudent investment compared to a range of generally accepted medical interventions. An estimated cost per quality-adjusted life year of approximately $20,000 to $40,000 compares favorably to those for a wide range of preventive and therapeutic services (34, 35), such as use of tissue plasminogen activator for myocardial reperfusion (36) and pharmacotherapy for hypercholesterolemia among patients at moderate risk of heart disease (37). Arguments regarding expanded access to effective mental health treatment have often been framed in terms of cost savings or cost offset. Unfortunately, this argument often cloaks a discriminatory assumption that effective mental health care is justifiable only if it reduces overall health care expenditures. We do not believe that expenditures for treatment of other major health conditions are held to such a standard. When judged by a more appropriate yardstick that includes improvements in health as well as dollars spent, improved depression treatment compares favorably with other uses of health care resources.
 |
 |
 |
 |
Received Sept. 21, 2000; revision received Feb. 13, 2001; accepted March 23, 2001. From the Center for Health Studies, Group Health Cooperative; the Department of Psychiatry and Behavioral Sciences and the Department of Biostatistics, University of Washington, Seattle; and the Center for Health Services Research, UCLA Neuropsychiatric Institute, Los Angeles. Address reprint requests to Dr. Simon, Center for Health Studies, Group Health Cooperative, 1730 Minor Ave., #1600, Seattle, WA 98101-1448; [email protected] (e-mail). Supported by NIMH grant MH-41739.
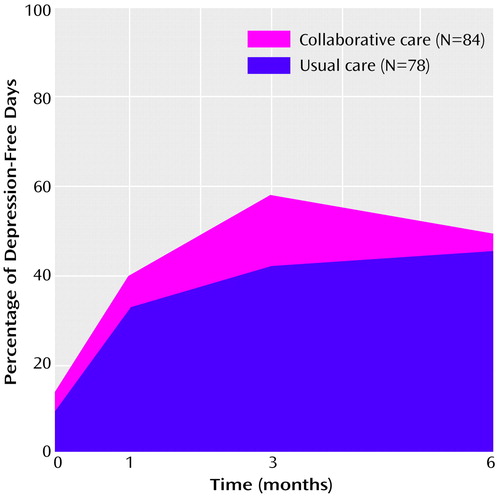
Figure 1. Mean Percentage of Depression-Free Days During 6-Month Follow-Up for Primary Care Patients Receiving Either Collaborative Care or Usual Care for Persistent Depressive Symptomsa
aDepression-free days were calculated as the area under the time curve of SCL-90 depression scores, with a score of 0.50 defined as “depression-free” and a score of 2.0 considered “fully symptomatic.”
1. Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV III, Hahn SR, Brody D, Johnson JG: Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. JAMA 1994; 272:1749-1756Crossref, Medline, Google Scholar
2. Ustun T, Sartorius N: Mental Illness in General Health Care. New York, John Wiley & Sons, 1995Google Scholar
3. Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, Berry S, Greenfield S, Ware J: The functioning and well-being of depressed patients: results from the Medical Outcomes Study. JAMA 1989; 262:914-919Crossref, Medline, Google Scholar
4. Ormel J, VonKorff M, Ustun TB, Pini S, Korten A, Oldehinkel T: Common mental disorders and disability across cultures. JAMA 1994; 272:1741-1748Crossref, Medline, Google Scholar
5. Spitzer R, Kroenke K, Linzer M, Hahn SR, Williams JBW, deGruy FV, Brody D, Davies M: Health-related quality of life in primary care patients with mental disorders. JAMA 1995; 274:1511-1517Crossref, Medline, Google Scholar
6. Schulberg H, Block M, Madonia M, Scott C, Lave J, Rodriguez E, Coulehan J: The “usual care” of major depression in primary care practice. Arch Fam Med 1997; 6:334-339Crossref, Medline, Google Scholar
7. Wells KB, Katon W, Rogers B, Camp P: Use of minor tranquilizers and antidepressant medications by depressed outpatients: results from the Medical Outcomes Study. Am J Psychiatry 1994; 151:694-700Link, Google Scholar
8. Katz S, Kessler R, Lin E, Wells K: Medication management of depression in the United States and Canada. J Gen Intern Med 1998; 13:77-85Crossref, Medline, Google Scholar
9. Schulberg HC, Block MR, Madonia MJ, Scott CP, Rodriguez E, Imber SD, Perel J, Lave J, Houck PR, Coulehan JL: Treating major depression in primary care practice: eight-month clinical outcomes. Arch Gen Psychiatry 1996; 53:913-919Crossref, Medline, Google Scholar
10. Katzelnick D, Simon G, Pearson S, Manning W, Helstad C, Henk H, Cole S, Lin E, Taylor L, Kobak K: Randomized trial of a depression management program in high utilizers of medical care. Arch Fam Med 2000; 9:345-351Crossref, Medline, Google Scholar
11. Katon W, VonKorff M, Lin E, Walker E, Simon GE, Bush T, Robinson P, Russo J: Collaborative management to achieve treatment guidelines: impact on depression in primary care. JAMA 1995; 273:1026-1031Crossref, Medline, Google Scholar
12. Katon W, Robinson P, VonKorff M, Lin E, Bush T, Ludman E, Simon G, Walker E: A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry 1996; 53:924-932Crossref, Medline, Google Scholar
13. Katon W, VonKorff M, Lin E, Simon G, Walker G, Unützer J, Bush T, Russo J, Ludman E: Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry 1999; 56:1109-1115Crossref, Medline, Google Scholar
14. Simon GE, VonKorff M, Rutter C, Wagner E: Randomised trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. BMJ 2000; 320:550-554Crossref, Medline, Google Scholar
15. Wells KB, Sherbourne C, Schoenbaum M, Duan N, Meredith L, Unützer J, Miranda J, Carney MF, Rubenstein LV: Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. JAMA 2000; 283:212-220Crossref, Medline, Google Scholar
16. Lave JR, Frank RG, Schulberg HC, Kamlet MS: Cost-effectiveness of treatments for major depression in primary care practice. Arch Gen Psychiatry 1998; 55:645-651Crossref, Medline, Google Scholar
17. Simon GE, Manning WG, Katzelnick DJ, Perarson SD, Henk HJ, Helstad CS: Cost-effectiveness of systematic depression treatment for high utilizers of general medical care. Arch Gen Psychiatry 2001; 58:181-187Crossref, Medline, Google Scholar
18. VonKorff M, Katon W, Bush T, Lin EH, Simon GE, Saunders K, Ludman E, Walker E, Unützer J: Treatment costs, cost offset, and cost-effectiveness of collaborative management of depression. Psychosom Med 1998; 60:143-149Crossref, Medline, Google Scholar
19. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version. Washington, DC, American Psychiatric Press, 1996Google Scholar
20. Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L: The Hopkins Symptom Checklist (HSCL): a measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry 1974; 7:43-45Google Scholar
21. VonKorff M, Wagner EH, Saunders K: A chronic disease score from automated pharmacy data. J Clin Epidemiol 1992; 45:197-203Crossref, Medline, Google Scholar
22. Clark D, VonKorff M, Saunders K, Baluch WM, Simon GE: A chronic disease score with empirically derived weights. Med Care 1995; 33:783-795Crossref, Medline, Google Scholar
23. Efron B: The Jackknife, the Bootstrap, and Other Resampling Plans. Philadelphia, Society for Industrial and Applied Mathematics, 1982Google Scholar
24. Stata Reference Manual: Release 6.0. College Station, Tex, Stata Corp, 1999Google Scholar
25. Kessler RC, Foster CL, Saunders WB, Stang PE: Social consequences of psychiatric disorders, I: educational attainment. Am J Psychiatry 1995; 152:1026-1032Link, Google Scholar
26. Berndt ER, Koran LM, Finkelstein SN, Gelenberg AJ, Kornstein SG, Miller IM, Thase ME, Trapp GA, Keller MB: Lost human capital from early-onset chronic depression. Am J Psychiatry 2000; 157:940-947Link, Google Scholar
27. Kessler RC, Walters EE, Forthofer MS: The social consequences of psychiatric disorders, III: probability of marital stability. Am J Psychiatry 1998; 155:1092-1096Link, Google Scholar
28. Wells K, Sherbourne C: Functioning and utility for current health of patients with depression or chronic medical conditions in managed, primary care practices. Arch Gen Psychiatry 1999; 56:897-904Crossref, Medline, Google Scholar
29. Unützer J, Patrick D, Diehr P, Simon G, Grembowski D, Katon W: Quality adjusted life years in older adults with depressive symptoms and chronic medical disorders. Int Psychogeriatr 2000; 12:15-33Crossref, Medline, Google Scholar
30. Revicki D, Wood M: Patient-assigned health state utilities for depression-related outcomes: differences by depression severity and antidepressant medications. J Affect Disord 1998; 48:25-36Crossref, Medline, Google Scholar
31. Kaplan R: Health-related quality of life in mental health services evaluation, in Cost-Effectiveness of Psychotherapy: A Guide for Practitioners, Researchers, and Policy-Makers. Edited by Miller N, Magruder K. New York, Oxford University Press, 1999, pp 213-228Google Scholar
32. Fryback D, Dasbach E, Klein R, Klein B, Dorn N, Peterson K, Martin P: The Beaver Dam Health Outcomes Study: initial catalog of health state quality factors. Med Decis Making 1993; 13:89-102Crossref, Medline, Google Scholar
33. Pyne J, Patterson T, Kaplan R, Ho S, Gillin J, Golshan S, Grant I: Preliminary longitudinal assessment of quality of life in patient with major depression. Psychopharmacol Bull 1997; 33:23-29Medline, Google Scholar
34. Gold M, Siegel J, Russell L, Weinstein M: Cost-Effectiveness in Health and Medicine. New York, Oxford University Press, 1996Google Scholar
35. Tengs T, Adams M, Pliskin J, Safran D, Siegel J, Weinstein M, Graham J: Five-hundred life-saving interventions and their cost-effectiveness. Risk Anal 1995; 15:369-390Crossref, Medline, Google Scholar
36. Mark D, Hlatky M, Califf R, Naylor C, Lee K, Armstrong P, Garbash G, White H, Simoons M, Nelson C, Clapp-Channing N, Knight J, Harrell F, Simes J, Topol E: Cost-effectiveness of thrombolytic therapy with tissue plasminogen activator as compared with streptokinase for acute myocardial infarction. N Engl J Med 1995; 332:1418-1424Crossref, Medline, Google Scholar
37. Goldman L, Weinstein M, Goldman P, Williams L: Cost-effectiveness of HMG-CoA reductase inhibition for primary and secondary prevention of coronary heart disease. JAMA 1991; 265:1145-1151Crossref, Medline, Google Scholar



