The Specificity of Depressive Symptoms in Patients With Alzheimer’s Disease
Abstract
OBJECTIVE: This study assessed the specificity of depressive symptoms in patients with Alzheimer’s disease and examined the discrepancies between patient and caregiver symptom reports. METHOD: The study group was composed of a series of 233 patients with Alzheimer’s disease, 47 patients with depression but without dementia, and 20 healthy comparison subjects; the latter two groups were comparable in age with the patients with Alzheimer’s disease. The patients and comparison subjects received a comprehensive psychiatric evaluation, which included administration of the Hamilton Depression Rating Scale and the Structured Clinical Interview for DSM-IV. RESULTS: Patients with Alzheimer’s disease with a score of 2 or higher on the “depressed mood” item of the Hamilton depression scale, as scored by their respective caregivers, comprised a group with depressed mood (N=92), whereas patients who scored 0 on this item comprised a group without depressed mood (N=62). A statistical comparison of the scores on the remaining Hamilton depression scale items (2–16) between the Alzheimer’s disease patients with and without depressed mood revealed significant differences on all items, except “loss of appetite.” However, there were no significant differences on any single Hamilton depression scale item between the Alzheimer’s disease patients without depressed mood and the age-comparable healthy comparison subjects. CONCLUSIONS: Depressive symptoms are not widespread among patients with Alzheimer’s disease but are significantly related to an underlying depressed mood. Patients with Alzheimer’s disease may not be fully aware of the extent of their depressive symptoms.
The nosological significance of depressed mood and depressive syndromes in patients with Alzheimer’s disease remains a controversial issue. Studies of depression in patients with Alzheimer’s disease performed during the last decade have produced wide discrepancies in estimation of prevalence, main clinical correlates, and response to treatment (1). These discrepancies may result from several methodological biases, such as assessment of depressive symptoms with a variety of psychiatric instruments (2–4), use of different strategies to diagnose depression, and reliance on information provided by either caregivers or patients (5, 6). To our knowledge, the specificity of depressive symptoms in Alzheimer’s disease has not been empirically assessed, although studies suggest that major depression and dysthymia in Alzheimer’s disease have different clinical correlates and a different longitudinal evolution (2).
The overall aim of the present study was to examine the phenomenology of depression in patients with Alzheimer’s disease. The specific aims of the study were to assess the specificity of depressive symptoms in patients with Alzheimer’s disease, to determine whether the phenomenology of depression in Alzheimer’s disease is similar to the profile of symptoms in primary (i.e., no known brain injury) depression, to examine whether depressed patients with Alzheimer’s disease have a poor awareness of their own depressive symptoms, and to determine the prevalence of “masked” depression in patients with Alzheimer’s disease (i.e., with symptoms sufficient to meet DSM-IV criteria for major or minor depression in the absence of depressed mood or loss of interest).
Method
Our initial group of subjects was a consecutive series of 233 patients with probable Alzheimer’s disease (7) who visited the dementia clinic at our facility because of progressive cognitive decline. The group with depression was composed of a consecutive series of 47 patients aged over 60 years who visited the psychiatric clinic at our facility because of a depressed mood. The inclusion criteria for this group included 1) a DSM-IV diagnosis of either major or minor depression and 2) meeting none of the DSM-IV clinical criteria for dementia. The healthy comparison group was composed of 20 individuals aged over 60 years; most were volunteers from our facility.
After the methodology of the study had been fully explained, written informed consent was obtained from the patients and their respective caregivers and from the healthy comparison subjects. The patients and healthy comparison subjects were assessed by a psychiatrist (who was blind to the purpose of the study) with the following instruments: the Structured Clinical Interview for DSM-III-R (SCID) (8), the Mini-Mental State (9), the Hamilton Depression Rating Scale (10), the Hamilton Anxiety Rating Scale (11), the Clinical Dementia Rating Scale (12), and the Apathy Scale (13).
A neurological examination consisted of administration of the Unified Parkinson’s Disease Rating Scale (14), in which results were assessed by a neurologist (J.K.) who was blind to psychiatric findings. The patients with Alzheimer’s disease were first interviewed by a psychiatrist (E.C.) with the use of all of the psychiatric instruments previously described except the SCID. Simultaneously, caregivers, who were blind to the results of these interviews, rated the patients’ behavior with the same instruments. Finally, the psychiatrist administered the SCID to each patient with both the patient and the caregiver present.
Statistical analysis was carried out with the use of means and standard deviations, two-way analysis of variance (ANOVA), and post hoc planned comparisons. Frequency distributions were analyzed by means of chi-square tests with a Yates’s correction for expected cell sizes of less than 5. All p values were two-tailed.
Results
On the basis of answers provided by caregivers to the “depressed mood” item on the Hamilton depression scale, the patients with Alzheimer’s disease were divided into two groups: 1) those with Alzheimer’s disease and depressed mood (N=92), which included patients with scores of 2 points or higher on this item, and 2) those with Alzheimer’s disease without depressed mood (N=62), which included patients with scores of 0 on this item. (Patients with a score of 1 [N=79] were excluded from further analysis in this study.)
Demographic and Clinical Findings
No significant between-group differences were found between patients with Alzheimer’s disease with and without depressed mood among demographic variables, severity of dementia ratings, or Mini-Mental State scores (Table 1). The depressed group with Alzheimer’s disease had significantly higher Hamilton depression scale total scores as rated by caregivers (t=11.3, df=152, p<0.0001) and the patients themselves (t=6.0, df=152, p<0.0001) than the nondepressed patients with Alzheimer’s disease. The depressed patients with Alzheimer’s disease also showed significantly higher scores for anxiety (Hamilton anxiety scale: t=4.4, df=152, p<0.0001), apathy (Apathy Scale: t=3.1, df=152, p<0.01), and parkinsonism (Unified Parkinson’s Disease Rating Scale, 22 patients not scored: t=4.5, df=130, p<0.0001) than the nondepressed patients with Alzheimer’s disease. Five (8%) of the 62 nondepressed patients with Alzheimer’s disease and 16 (17%) of the 92 depressed patients with Alzheimer’s disease were being treated with antidepressants.
Psychiatric Findings
To examine the differences on individual Hamilton depression scale items among the four study groups, we calculated a two-way ANOVA (four groups times 15 Hamilton depression scale items). There was a significant main effect (F=53.3, df=3, 127, p<0.0001) and a significant group-by-item interaction effect (F=5.4, df=42, 3038, p<0.0001). On individual comparisons, a significant main effect was found for all 15 Hamilton depression scale items (Figure 1). (Hamilton depression scale items 1 and 17 were not included in the ANOVA but are shown in Figure 1). The depressed patients with Alzheimer’s disease had significantly higher scores than the nondepressed patients with Alzheimer’s disease on the following Hamilton depression scale items: guilt (F=24.7, df=1, 152, p<0.0001), suicide (F=16.0, df=1, 152, p<0.0001), early insomnia (F=12.0, df=1, 152, p<0.001), middle insomnia (F=13.8, df=1, 152, p<0.001), late insomnia (F=11.2, df=1, 152, p<0.001), loss of interest (F=17.1, df=1, 152, p<0.0001), psychomotor retardation (F=23.6, df=1, 152, p<0.0001), agitation (F=11.6, df=1, 152, p<0.001), worry (F=37.4, df=1, 152, p<0.0001), anxiety (F=20.6, df=1, 152, p<0.0001), loss of energy (F=22.4, df=1, 152, p<0.0001), loss of libido (F=9.01, df=1, 152, p<0.01), hypochondriasis (F=24.0, df=1, 152, p<0.0001), and loss of weight (F=8.9, df=1, 152, p<0.01). No significant differences among groups were found for loss of appetite. (“Sadness” and “loss of insight” were not included in the ANOVA.) Healthy comparison subjects and nondepressed patients with Alzheimer’s disease showed no significant between-group differences for any Hamilton depression scale item. Finally, a comparison between the depressed patients with Alzheimer’s disease and the depressed patients without dementia demonstrated significantly higher scores for the latter on the following Hamilton depression scale items: suicide (F=33.4, df=1, 137, p<0.0001), anxiety (F=8.8, df=1, 137, p<0.01), loss of appetite (F=22.3, df=1, 137, p<0.0001), and loss of weight (F=19.1, df=1, 137, p<0.0001). However, the depressed patients with Alzheimer’s disease had significantly higher scores on psychomotor retardation (F=11.8, df=1, 137, p<0.001) than the depressed patients without dementia.
Diagnoses of major and minor depression were generated on the basis of SCID assessments. The frequencies of major depression were much higher for the depressed patients with Alzheimer’s disease than for the nondepressed patients with Alzheimer’s disease, whereas the frequencies for minor depression were somewhat higher for the same group. Only 4% (N=4) of the depressed patients with Alzheimer’s disease failed to meet criteria for either major or minor depression compared to 68% (N=42) of the nondepressed patients with Alzheimer’s disease. Among all patients with Alzheimer’s disease, a hypothesis of unequal frequency of minor, major, or no depression based on the presence of depressed mood (i.e., a score of 2 or higher on the “depressed mood” item on the Hamilton depression scale) was statistically substantiated (χ2=78.9, df=2, p<0.0001). A score of 2 or higher on the same item (as rated by the caregiver) had a sensitivity of 61% and a specificity of 94% for major depression, whereas the patients’ own ratings on this item had a sensitivity of 46% (42 of the 92 depressed patients with Alzheimer’s patients had a score of 2 or higher) and a specificity of 94% (four of the 62 nondepressed patients with Alzheimer’s disease had a score of 2 or higher).
“Masked” Depression
We also examined whether some patients with Alzheimer’s disease had “masked” major depression (i.e., four or more of the DSM-IV criteria for major depression, without depressed mood or loss of interest). Only one (2%) of the 62 patients without depressed mood met this condition. However, “masked” minor depression was significantly more frequent; 11 (18%) of the 62 nondepressed patients with Alzheimer’s disease met at least one criterion for minor depression in the absence of depressed mood or loss of interest (χ2=7.5, df=1, p<0.01).
Awareness of Depression
To examine the awareness of depressive symptoms in depressed patients with Alzheimer’s disease, we compared Hamilton depression scale ratings obtained from patient interviews with those obtained from their respective caregivers. A two-way repeated measures ANOVA (caregiver-by-item) showed a significant effect for caregiver (F=61.8, df=1, 282, p<0.0001). Caregivers rated patients as significantly more depressed than did the patients themselves. In addition, there was a significant informant-by-item interaction effect (F=12.4, df=14, 3920, p<0.0001). On the other hand, only 3% (N=3) of the patients with Alzheimer’s disease diagnosed on the basis of information from caregivers denied having a depressed mood.
Discussion
This study examined the prevalence of depressive symptoms among patients with Alzheimer’s disease; there were several important findings. First, depressed patients with Alzheimer’s disease (as defined by a score of 2 points or higher on the “depressed mood” item on the Hamilton depression scale, as scored by a caregiver) had significantly higher scores on most items of the Hamilton depression scale than did nondepressed patients with Alzheimer’s disease. Second, depressed patients with Alzheimer’s disease had a profile of depressive symptoms similar to those of age-matched patients with primary depression without dementia. Third, nondepressed patients with Alzheimer’s disease had no more symptoms of depression than did age-matched healthy comparison subjects, demonstrating that dementia (at least in the mild to moderate stages) does not produce the symptoms of depression in the absence of depressed mood. Fourth, “masked” major depression (i.e., meeting four or more DSM-IV criteria for major depression without depressed mood or loss of interest) was present in only 1.6% of the nondepressed patients with Alzheimer’s disease, confirming that symptoms of depression are not rampant among nondepressed patients with Alzheimer’s disease. Finally, depressed patients with Alzheimer’s disease rated their depression (on the Hamilton depression scale) as less severe than did their respective caregivers, suggesting that depressed patients with Alzheimer’s disease may not be fully aware of the severity of their depressive symptoms.
An issue under debate is how to diagnose depression in the context of a chronic neurological illness that involves cognitive decline and prominent behavioral changes. We found that depressed mood in Alzheimer’s disease was significantly associated with a range of depressive symptoms—guilt, suicide, insomnia, loss of interest, retardation, agitation, worry, anxiety, loss of energy, loss of libido, loss of weight, and hypochondriasis—which suggests that both affective and autonomic symptoms of depression are frequent among patients with Alzheimer’s disease. We found a similar prevalence of depressive symptoms among depressed patients with Alzheimer’s disease and patients with primary depression without dementia, except for loss of appetite, loss of weight, suicide, and anxiety, which were more severe in the latter. To examine whether depressive symptoms in dementia are nonspecific manifestations of a chronic neurological disorder, we compared the severity of depressive symptoms in nondepressed patients with Alzheimer’s disease with those of age-matched healthy comparison subjects. There were no significant differences in the frequency of any of the symptoms, which demonstrates that both affective and autonomic symptoms of depression are not epiphenomena of a chronic neurologic disease but specific symptoms of a mood disorder.
A question now arising is whether patients with Alzheimer’s disease may show “masked” depression (i.e., depressive symptoms in the absence of depressed mood). We found that only 2% of the nondepressed patients with Alzheimer’s disease met four of the DSM-IV criteria for major depression in the absence of depressed mood or loss of interest. On the other hand, 18% of the nondepressed patients with Alzheimer’s disease met the criteria for minor depression except for depressed mood or loss of interest. This may result from the fact that the diagnosis of minor depression requires meeting only one criterion for major depression, plus depressed mood or loss of interest. Several studies have demonstrated important differences between major depression and the dysthymia found in Alzheimer’s disease. Major depression usually begins before the onset of cognitive decline (2), is associated with poor awareness of intellectual deficits (2), is related to cerebral perfusion deficits in specific brain areas (15), and has a significantly longer duration than dysthymia (16). On the other hand, dysthymia most often begins after the onset of dementia (2), has a higher prevalence in the early stages of the illness (2), is related to the relative preservation of awareness of cognitive impairment (2), and is not associated with specific changes in cerebral perfusion (15). On the basis of the previous findings, we (17) have proposed that major depression found in Alzheimer’s disease may be related to biological factors, whereas dysthymia may be an emotional reaction of predisposed individuals to progressive cognitive decline. Nonetheless, minor depression and dysthymia have different diagnostic criteria, and future studies should examine whether these diagnoses share similar clinical correlates in Alzheimer’s disease.
In the present study, we also examined the awareness of depressive symptoms in patients with Alzheimer’s disease. We found that depressed patients with Alzheimer’s disease significantly underrated the severity of their depressive symptoms compared to reports provided by their respective caregivers, which emphasizes the importance of questioning both patients and caregivers for the presence of depressive symptoms. On the other hand, the patients with Alzheimer’s disease were able to provide valid information about their mood state, since only 3% of the patients with Alzheimer’s disease with major depression diagnosed on the basis of information from caregivers denied having a depressed mood.
In conclusion, our study demonstrated that affective and autonomic symptoms of depression are both related to the presence of a depressed mood in patients with Alzheimer’s disease and should not be considered nonspecific epiphenomena of cognitive decline. Future studies should examine the specificity and clinical correlates of major depression, minor depression, and dysthymia in patients with Alzheimer’s disease.
 |
Received Sept. 29, 1999; revisions received April 10 and July 14, 2000; accepted July 24, 2000. From the Department of Neuropsychiatry, Raúl Carrea Institute of Neurological Research, Buenos Aires; and the Department of Psychiatry, University of Iowa School of Medicine, Iowa City. Address reprint requests to Dr. Starkstein, FLENI, Montañeses 2325, 1428 Buenos Aires, Argentina; [email protected] (e-mail). Supported in part by grants from the Raúl Carrea Institute of Neurological Research, the Fundación Perez Companc, and the CONICET.The authors thank Paul Fedoroff, M.D., for his help.
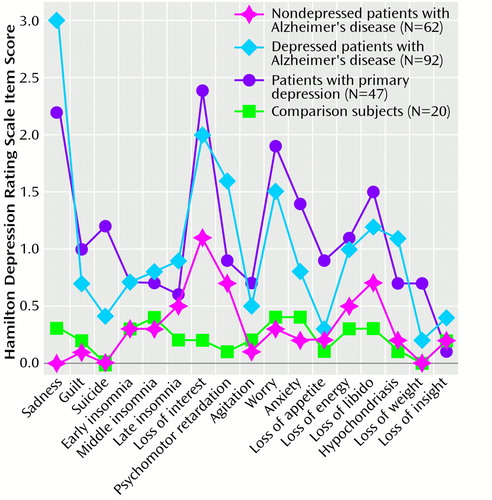
Figure 1. Hamilton Depression Scale Item Scores of Depressed and Nondepressed Patients With Alzheimer’s Disease, Depressed Patients Without Dementia, and a Healthy Comparison Groupa
aStatistically significant difference on all 17 items (ANOVA, p<0.01).
1. Wragg RE, Jeste DV: Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 1989; 146:577–587Link, Google Scholar
2. Migliorelli R, Tesón A, Sabe L, Petracchi M, Leiguarda R, Starkstein SE: Prevalence and correlates of dysthymia and major depression among patients with Alzheimer’s disease. Am J Psychiatry 1995; 152:37–44Link, Google Scholar
3. Brodaty H, Luscombe G: Depression in persons with dementia. Int Psychogeriatr 1996; 8:609–622Crossref, Medline, Google Scholar
4. Teri L, Logsdon RG, Uomoto J, McCurry SM: Behavioral treatment of depression in dementia patients: a controlled clinical trial. J Gerontol B Psychol Sci Soc Sci 1997; 52:159–166Crossref, Google Scholar
5. Burns A, Jacoby R, Levy R: Psychiatric phenomena in Alzheimer’s disease, III: disorders of mood. Br J Psychol 1990; 157:81–86Crossref, Google Scholar
6. Mackenzie TB, Robiner WN, Knopman DS: Differences between patient and family assessments of depression in Alzheimer’s disease. Am J Psychiatry 1989; 146:1174–1178Google Scholar
7. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
8. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
9. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
10. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
11. Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
12. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566–572Crossref, Medline, Google Scholar
13. Starkstein SE, Migliorelli R, Manes F, Tesón A, Petracca G, Chemerinski E, Sabe L, Leiguarda R: The prevalence and clinical correlates of apathy and irritability in Alzheimer’s disease. Eur J Neurol 1995; 2:1–7Crossref, Google Scholar
14. Fahn S, Elton E (UPDRS Development Committee): Unified Parkinson’s disease rating scale, in Recent Developments in Parkinson’s Disease. Edited by Fahn S, Marsden CD, Goldstein M, Calne CD. Florham Park, NJ, Macmillan, 1987, pp 153–163Google Scholar
15. Starkstein SE, Vazquez S, Migliorelli R, Tesón A, Petracca G, Leiguarda R: A SPECT study of depression in Alzheimer’s disease. Neuropsychiatry Neuropsychol Behav Neurol 1995; 8:38–43Google Scholar
16. Starkstein SE, Chemerinski E, Sabe L, Kuzis G, Petracca G, Tesón A, Leiguarda R: Prospective longitudinal study of depression and anosognosia in Alzheimer’s disease. Br J Psychiatry 1997; 171:47–52Crossref, Medline, Google Scholar
17. Starkstein SE: Neurological models of depression. Adv Biol Psychiatry 1999; 19:123–135Crossref, Google Scholar



