Saccadic Disinhibition in Patients With Acute and Remitted Schizophrenia and Their First-Degree Biological Relatives
Abstract
OBJECTIVE: Performance on measures of saccadic inhibition and control was investigated in a large family study of schizophrenia to evaluate the utility of using antisaccade task performance as an endophenotypic marker of genetic liability for schizophrenia. METHOD: Ninety-five patients with acute schizophrenia and 116 of their first-degree biological relatives, 13 schizophrenia patients whose illness was in full remission, 35 patients with acute psychotic affective disorder, and 109 nonpsychiatric comparison subjects were administered antisaccade and prosaccade tasks. RESULTS: Both schizophrenia patient groups had a greater number of errors on the antisaccade task than did the first-degree relatives and the affective disorder group, which both had more errors than the comparison subjects. Among the first-degree relatives of the probands with acute schizophrenia, relatives of poor-performing patients performed worse on the antisaccade task than relatives of patients with good performance. Reflexive errors were not likely the result of interfering psychotic symptoms, medication, or medication side effects. Although the schizophrenia patients demonstrated other signs of saccadic abnormalities, these problems, which were not observed in their relatives even though they had high antisaccade error rates, seem unlikely to account for the higher antisaccade error rate of the schizophrenia patients. CONCLUSIONS: These findings suggest that saccadic disinhibition is strongly associated with the genetic liability for schizophrenia.
Antisaccade task performance may be a useful indicator of prefrontal integrity as well as an endophenotypic marker of genetic liability for schizophrenia. An endophenotype refers to some neurobiological indicator that is more closely related to the genes causing the predisposition for a disease than the symptoms that lead to the diagnosis of the disease. Antisaccade tasks (1) measure oculomotor response inhibition and require voluntary control over prepotent reflexive saccades. Reflexive errors made during antisaccade tasks occur when one has difficulty inhibiting saccades under conditions where they are context-inappropriate. Several studies have demonstrated that schizophrenia patients, like various neurological patient groups, generate a greater number of reflexive errors on antisaccade tasks (2–7). Neurological patient investigations (8, 9) and neuroimaging data (10–13) have led to the hypothesis that reflexive errors in schizophrenia are the result of prefrontal cortical dysfunction.
In addition to providing useful leads regarding the pathophysiology of schizophrenia, studies of antisaccade task performance may also provide indications of genetic liability for schizophrenia and identification of potential gene carriers. Several studies have demonstrated that the relatives of probands with schizophrenia show a higher rate of reflexive errors on antisaccade tasks (2, 7, 14, 15). McDowell et al. (15) reported that relatives selected from families in which multiple members were affected with schizophrenia had worse antisaccade performance than relatives from families where only one member of the family was affected. In addition, first-degree relatives were more likely to perform poorly than second-degree relatives of probands with schizophrenia. These results indicate that difficulty inhibiting inappropriate saccades is associated with an increasing degree of genetic loading for schizophrenia. A recent study failed to find a significant difference between relatives and nonpsychiatric comparison subjects on antisaccade performance but did report that the relatives of patients with poor performance had worse antisaccade performance than the relatives of patients with normal performance (16).
An endophenotypic marker for genetic risk ideally has a reasonable degree of diagnostic specificity (17). While worse antisaccade performance has been reported in psychiatric disorders other than schizophrenia, the meaning of these findings is unclear. Obsessive-compulsive disorder (OCD) (18, 19), bipolar disorder (7, 20, 21), and major depression (22) have all been linked to poor antisaccade task performance. However, these studies have demonstrated that the degree of antisaccade performance deficit is greater in patients with schizophrenia relative to those with other psychiatric disorders. In addition, some studies have found that patients with OCD (14), unipolar depression (7), and bipolar disorder (2, 4, 14, 23) do not have difficulties with antisaccade tasks. Inconsistencies may be due to the inclusion of patients with and without psychotic features and relatively small sample sizes. Nonetheless, it could be the case that individuals with different psychiatric disorders who perform poorly on antisaccade tasks share a common pathophysiology, possibly localized to the prefrontal cortex. Indeed, data exist showing that each of the above psychiatric conditions shows signs of prefrontal dysfunction.
The current study has several goals. To our knowledge, this is the largest study of antisaccade performance in biological relatives of schizophrenia patients. (The study is comparable with the McDowell et al. report [15], which pooled relatives from three independent samples.) Unlike other studies with smaller study group sizes, this investigation addressed the effects of including relatives with psychiatric disorders. Relatives of probands with schizophrenia have been shown to have a higher incidence of various psychiatric disorders—such as psychotic affective disorders (24)—and these conditions can result in higher antisaccade error rates (22). Thus, our first goal was to demonstrate the presence of antisaccade deficits in relatives who were free of psychiatric disease. Otherwise, reported differences may be related to the presence of psychiatric symptoms instead of proposed genetic risk.
Second, it is possible that symptoms of acute psychosis disrupt the ability of patients with schizophrenia to concentrate and perform well on antisaccade tasks. If reflexive errors on antisaccade tasks represent a valid trait marker of prefrontal dysfunction in schizophrenia, then they should be present even during remission. Since there appear to be no studies to date demonstrating that this deficit is present during symptom remission, we included a group of schizophrenia patients whose illness was in full remission. In addition, within the patient groups, the association between medication and extrapyramidal side effects and other aspects of saccadic control was analyzed.
Third, to examine diagnostic specificity and the general effects of psychosis, saccade performance was collected from mood disorder patients who were included only if they had current psychotic features. And finally, a visually guided prosaccade task was included to further evaluate the control of triggering and accuracy of saccades. Such analyses are important because the specificity of saccadic problems has implications for making inferences about neuropathology.
Method
Participants
Ninety-five patients in an acute episode of schizophrenia (64 men and 31 women; mean age=35 years, SD=9.7; mean Global Assessment of Functioning Scale score=26.0, SD=9.3) were recruited from an inpatient unit of a hospital that serves a large metropolitan area. We also recruited 116 subjects (50 men and 66 women; mean age=42 years, SD=11.9) who were biological first-degree relatives of 44 of the probands with acute schizophrenia (2.6 relatives per proband). Thirteen individuals with schizophrenia in full remission (eight men and five women; mean age=48 years, SD=12.8; mean Global Assessment of Functioning Scale score=65.3, SD=6.7) were recruited from the outpatient department of the study hospital. Remission was defined as a DSM-IV diagnosis of schizophrenia in remission and a rating <30 on the Brief Psychiatric Rating Scale (BPRS) (25). This patient group had a mean BPRS score of 21.2 (SD=2.3), which is only slightly above the minimum score of 18 that can be obtained on the BPRS. For schizophrenia to be considered remitted, patients also had to have a rating <3 on the affective flattening subscale of the Scale for the Assessment of Negative Symptoms (SANS) (26) and ratings of <2 on the other four scales (alogia, avolition, anhedonia, and attention). Additionally, they had to have a rating >60 on the Global Assessment of Functioning Scale, indicating high overall functioning. Finally, these subjects had to have been free of psychotic symptoms for at least 1 month, not have been hospitalized for psychiatric problems for the past 3 months, have at most only one residual symptom, and currently work and associate with friends.
Two comparison groups were included. Thirty-five patients with an affective disorder (major depressive disorder, N=13; bipolar disorder, N=22) with current psychotic features participated (16 men and 19 women; mean age=31 years, SD=10.6; mean Global Assessment of Functioning Scale score=26.8, SD=9.7). The second comparison group consisted of 109 nonpsychiatric participants (47 men and 62 women; mean age=35, SD=12.9) who were recruited from family practice and other nonpsychiatric medical clinics, trade schools, and churches. They were interviewed and included if they had never had a DSM-IV mood disorder, experienced a psychotic symptom, or reported lifetime substance dependence or current substance abuse. They were excluded if they reported that they or a first-degree biological relative had ever received treatment for any psychiatric disorder.
All participants were between the ages of 18 and 65, spoke English fluently, had not recently undergone ECT treatment, and had no history of neurological disease, systemic disease known to involve central nervous system functioning, clinically significant head injury, or mental retardation. All participants provided written informed consent. Diagnostic information was obtained by using the Structured Clinical Interview for DSM-IV (SCID, modules A–E) and chart reviews. The SCID was also used for assessing diagnoses in the nonpatient groups. To confirm diagnostic assignments, a consensus diagnostic team composed of doctoral-level students and clinical psychologists reviewed SCID, chart data, and audio recordings of interviews when necessary. A reliability study performed on a group of 58 patients with various diagnoses yielded a high level of diagnostic reliability (kappa=0.83). Additionally, all diagnostic and clinical ratings were made blind to saccadic functioning.
All patients were currently taking psychiatric medications that fell into six classes: typical antipsychotics (N=37), atypical antipsychotics (N=73), mood stabilizers (N=66), antidepressants (N=29), anxiolytics (N=25), and anticholinergics (N=26). Trained technicians assessed medication side effects in each of the patients. Widely used rating scales of extrapyramidal side effects, tardive dyskinesia, and akathisia (27–29) were used to measure the presence and extent of these medication side effects.
Saccadic Tasks
Ocular motor recordings were obtained in a quiet, darkened room by means of both monocular infrared and electro-oculographic (EOG) recording techniques. Head movement was minimized with use of a bite bar and dental impression. The eye-tracking measures were derived from the infrared recordings. Vertical EOG recordings were, however, used to aid in the identification and removal of blinks from the infrared record, since without the aid of EOG, blinks can masquerade as saccades. All stimuli (yellow circles, 0.5 degrees in diameter) were presented on a darkened computer monitor positioned 48 cm from the eyes of the participant.
In the antisaccade task (Figure 1), the target began at a central fixation point. Following a 2–3-second pseudorandom interval, the center fixation stimulus was extinguished and a peripheral cue simultaneously appeared 10 degrees either to the left or to the right in an unpredictable fashion. The cue remained at the periphery for 1.5 seconds. Subjects were instructed not to look at the cue but instead to direct their gaze to the opposite side. The cue then returned to central fixation, signaling the beginning of a new trial. Twenty trials were presented, 10 in which the stimulus appeared on the left and 10 in which it was on the right. The percentage of reflexive errors (inappropriate prosaccades) out of all valid trials, as well as initial saccadic reaction times, were computed. Subjects were given a short set of practice trials in order to confirm that they understood the instructions. Although rare, trials in which a reflexive error was made and a corrective antisaccade was not were excluded from analyses to assure us that trials in which the subject was not on task did not bias results.
Saccadic reaction times were also derived from a visually guided prosaccade task (Figure 1), which consisted of 23 trials in which the stimulus appeared 5 (N=7), 10 (N=11), 15 (N=2), and 20 (N=3) degrees to the left or right of the central fixation point in an unpredictable fashion. Twelve of these displacements were in the rightward direction. In addition, saccadic gain was used to estimate the accuracy of initial prosaccades. Gain was calculated as the amplitude of the initial saccade made to the visual target divided by the displacement of the target. Thus, it is a ratio of the eye amplitude to the target amplitude, with gains approaching 1.0 indicative of greater accuracy.
Results
Preliminary Analyses
Logarithmic transformations were performed on the saccadic reaction times to reduce skewness and heteroscedasticity. Gender (χ2=18.0, df=4, p<0.001) and age (F=13.63, df=4, 362, p<0.001) differed across groups. There were more men in the schizophrenia groups than in the other three groups, which did not differ from one another in terms of gender. However, since across- and within-group analyses of variance (ANOVAs) indicated that gender was unrelated to the performance variables derived from the antisaccade and prosaccade tasks, gender was henceforth ignored. The first-degree relatives and the patients with schizophrenia that was in remission were older than the other groups, which did not differ in age. As expected, age correlated modestly with all three of the saccadic reaction time variables (prosaccade: r=0.17, df=367, p<0.002; antisaccade [correct trials]: r=0.19, df=359, p<0.001; antisaccade [error trials]: r=0.24, df=358, p<0.0001). However, age did not correlate with the percentage of reflexive errors on the antisaccade task (r=–0.09, df=367, p>0.15) nor with prosaccade amplitude gain (r=–0.02, df=367, n.s.). Thus, age was considered only in analyses that involved saccadic reaction times.
Group Effects
Because other studies (e.g., references 7, 20) have indicated that patients with bipolar disorder but not patients with unipolar depression evince a higher number of reflexive errors on antisaccade tasks, we compared the oculomotor performance of both affective disorder groups. Percent error on antisaccade tasks did not differentiate the bipolar disorder patients (mean=39.3%, SD=16.4%) from those with unipolar depression (mean=33.2%, SD=22.7%) (F=0.83, df=1, 32, p=0.37). In addition, no significant differences emerged for the other oculomotor variables between the two affective groups after we controlled for age. Thus, patients with unipolar depression and bipolar disorder were combined into a single affective disorder group for all further analyses.
Antisaccade Errors
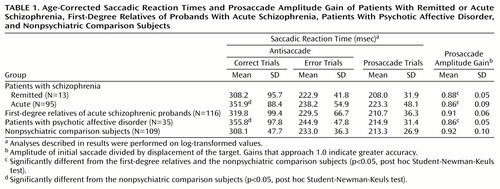
Groups differed significantly on the percentage of reflexive errors (F=24.89, df=4, 362, p<0.0005). Student-Newman-Keuls post hoc tests, with an alpha level of 0.05, indicated that the patients with remitted and acute schizophrenia made more mean errors (57.7% [SD=27.3%] and 55.6% [SD=26.0%], respectively) than the first-degree relatives (38.2% [SD=22.3%]) and the affective group (37.7% [SD=18.7%]), which both made more errors than the nonpsychiatric comparison group (24.6% [SD=17.0%]) (Figure 2). The patients with acute and remitted schizophrenia showed a similar reduced ability to inhibit unwanted saccades even though their clinical states were markedly different. The patients with psychotic affective disorder generated significantly fewer antisaccade errors than both schizophrenia patient groups.
Next, we compared the performance of the first-degree relatives on the basis of the proband’s performance on the antisaccade task. Note that this analysis excluded eight relatives who had a history of, but not current, psychotic diagnosis. The proband group was split at the percent error point that was two standard deviations worse than that of the mean for the nonpsychiatric group (cutoff=59%). Those below the cutoff (N=20) were classified as having good performance; those above the cutoff (N=24) were classified as having poor performance. The relatives (N=57) of probands with good performance had significantly fewer reflexive errors (mean=33.5%, SD=18.9%) than the relatives (N=51) of probands with poor performance (mean=43.3%, SD=25.4%) (t=2.24, df=107, p<0.03). At this two-standard-deviation cutoff, 46.2% (N=6) of the schizophrenia patients whose illness was in remission, 46.3% (N=44) of the patients with acute schizophrenia, 19.4% (N=21 of 108) of the first-degree relatives, 8.6% (N=3) of the patients with psychotic affective disorder, and 4.6% (N=5) of the nonpsychiatric comparison subjects could be classified as having impaired antisaccade performance. In addition, relatives of the schizophrenia probands with poor antisaccade performance were more likely to have impaired antisaccade performance than the relatives of probands with good antisaccade performance (χ2=41.7, df=1, p<0.001).
Saccadic Reaction Times
The only significant group effect, controlling for age, on the three saccadic reaction time measures was on saccadic reaction times during correct antisaccade trials (F=6.19, df=4, 353, p<0.001). Student-Newman-Keuls post hoc tests, with an alpha level of 0.05, were computed by using the age-corrected mean saccadic reaction times. The patients with acute schizophrenia or psychotic affective disorder, relative to the nonpsychiatric comparison group, had significantly increased latencies on correct trials. No other group had saccadic reaction times that were significantly different from the nonpsychiatric comparison subjects (Table 1). Within-group paired t tests indicated that all groups had significantly longer reaction times on correct antisaccade trials than on prosaccade trials and error antisaccade trials (all p<0.001). All groups had reaction times on error antisaccade trials that were significantly longer (p<0.01) than those of the prosaccade trials, except for the schizophrenia patients whose illness was in remission (t=1.57, df=12, p=0.14).
Amplitude Gain
Groups differed on prosaccade amplitude gain (F=13.85, df=4, 362, p<0.0005). Student-Newman-Keuls post hoc tests with an alpha level of 0.05 were computed. All three patient groups had a significantly lower amplitude gain than the first-degree relatives and nonpsychiatric comparison groups, which did not differ significantly from each other (Table 1). None of the patient groups differed significantly from one another on this measure.
Within-group correlations were also computed between the percentage of reflexive errors on the antisaccade task and gain. No significant associations were found (r=–0.12 to 0.09, all p>0.21). In addition, an analysis of covariance, with amplitude gain as a covariate, indicated that the significant group effects remained.
Medication Effects
To examine if medication side effects might be causing the oculomotor deficits, all patients (seven with acute schizophrenia and three with psychotic affective disorder) who had ratings of extrapyramidal side effects, tardive dyskinesia, or akathisia greater than “Questionable” were excluded. The aforementioned group effects, without exception, remained the same.
We performed multivariate analyses of variance (MANOVAs) for each of the six medication classes, with medication status (present/absent) as the independent variable and the saccadic measures as dependent variables. Neither typical nor atypical antipsychotic medication status was related to any of the dependent variables. Similarly, antidepressant and anticholinergic medication statuses were unrelated to any of the oculomotor measures. However, patients who were taking anxiolytics had lower prosaccade amplitude gain (F=11.08, df=1, 135, p<0.002). Patients taking anxiolytics were not significantly different on any of the other saccadic variables. Therefore, the group effects on antisaccade performance cannot be attributed to medication effects.
However, as anxiolytics may be related to hypometricity, we tested whether this could explain the three patient groups showing evidence of reduced saccadic amplitude gain. Thus, the MANOVA and follow-up tests were recomputed excluding all patients taking anxiolytics. The results remained the same.
Psychiatric Status Effects Among the First-Degree Relatives
Eight relatives had a past, but not current, psychotic diagnosis (e.g., schizophrenia, bipolar, delusional disorder) and 36 had other nonpsychotic axis I diagnoses (e.g., depression or substance dependence). Key analyses were repeated with the relatives with psychotic and other axis I diagnoses excluded to test whether the group differences could be attributed to psychiatric status. There were no differences between the healthy relative group and the comparison group on any of the saccadic reaction time variables or gain. However, the psychiatrically healthy relatives made a greater percentage of antisaccade errors than the nonpsychiatric comparison subjects (F=25.04, df=1, 176, p<0.001). Of importance, the performance of the relatives with past psychotic diagnoses on the antisaccade task (mean=48.1% errors, SD=33.9%) was more similar to that of the schizophrenia patients than to that of the healthy relatives (mean=39.4% errors, SD=24.5%).
Many of the participants and hence observations from our study were not independent. Some of the relatives came from the same family, and the relatives in general were selected because of their genetic relationship to the proband with schizophrenia. Thus, the denominator degrees of freedom used to derive p values were adjusted by replacing the number of individuals with the number of families. When this conservative procedure was applied, all of the preceding significant analyses remained significant (p<0.05).
Discussion
The results of the current study demonstrate that individuals selected solely because they have a first-degree relative with schizophrenia evidence robust difficulties inhibiting unwanted saccades. Even relatives who were free of lifetime axis I disorders demonstrated saccadic disinhibition. In addition, the relatives of probands with schizophrenia who performed poorly on the antisaccade task performed worse than the relatives of probands who had normal antisaccade task performance. Therefore, antisaccade task performance has obvious potential as an endophenotypic marker of genetic risk for schizophrenia and perhaps reflects prefrontal functioning. This is consistent with other data (14, 16) and suggests that antisaccade performance likely indexes the transmission of a gene or genes that are associated with the liability for developing neurocognitive dysfunction associated with schizophrenia. Indeed, a recent study of eight families with multiple cases of schizophrenia reported linkage (lod score=3.55) between a composite index of inhibition, which included antisaccade performance, and chromosome 22q (30).
Schizophrenia patients performed significantly worse on the antisaccade task than the psychotic affective disorder patients. However, the patients with unipolar depression and bipolar disorder also performed more poorly than nonpsychiatric participants. This finding leaves unresolved whether the affective patients’ deficit reflects a temporary or lasting characteristic. This issue could be resolved by evaluating the performance of the healthy relatives of these patients to determine if they too perform poorly. Another method of addressing the specificity of antisaccade deficits as a marker of genetic risk for schizophrenia would be to investigate whether affective patients still have difficulties with antisaccade tasks even when their illness is in remission. Again, failure to find antisaccade deficits in remitted affective disorder patients suggests that the deficits reported here are state-related.
Our findings indicate that regardless of clinical state, individuals with schizophrenia have significant difficulties inhibiting unwanted saccades. It is striking that the schizophrenia patients whose illness was in full remission continued to show reflexive errors at rates no different from acutely ill patients. It is unlikely, therefore, that psychotic symptoms or the severity of current clinical state can account for the greater number of reflexive errors seen in patients with schizophrenia.
Furthermore, it is highly unlikely that the antisaccade deficits reported here are due to detrimental effects of medications. Some studies have reported that antisaccade errors are more pronounced among patients who are taking neuroleptics (3) or show signs of tardive dyskinesia (31). In the current study, there was a low incidence of medication side effects, and analyses were performed with the few patients with notable side effects excluded. Even then, the schizophrenia patients showed comparable evidence of antisaccade errors. Also, it has been shown elsewhere that neuroleptic-free schizophrenia patients demonstrate higher rates of antisaccade errors (4). Nonetheless, it is possible that severe extrapyramidal side effects or tardive dyskinesia could affect antisaccade performance, but because of the low incidence of side effects in the patients, this study could not address such concerns. Moreover, the relatives who were not taking antipsychotics had significant difficulties inhibiting reflexive glances to the cue.
Other measures of saccadic control were assessed in this study to investigate whether generalized problems with saccadic control could underlie the higher rate of antisaccade errors reported in schizophrenia patients. Consistent with several other investigations (e.g., references 2, 3), schizophrenia patients showed mild difficulties generating reflexive saccades to visual targets. The schizophrenia and affective disorder patients demonstrated a greater frequency of visually guided saccades that were hypometric (fell slightly short of intended target). Nonetheless, caution must be used when interpreting these results. This finding was only found in the three patient groups. Moreover, it was found that some medications might have had detrimental effects upon patients’ ability to accurately execute correct amplitude saccades. Thus, it is unclear whether schizophrenia patients do indeed have problems making correct amplitude saccades to visual stimuli or whether this effect is due to psychiatric medications. The current study was not designed to be a study of medication effects on oculomotion, so more definitive conclusions cannot be drawn.
Despite the hypometria observed in the patients, it is unlikely that such a problem could be the cause of the greater number of reflexive errors reported among schizophrenia patients on the antisaccade task. This is especially true for the relatives, who had normal saccade metrics in all areas except in their ability to inhibit unwanted saccades. Hypometricity was unrelated to the percentage of reflexive antisaccade errors. Therefore the antisaccade deficits noted in schizophrenia are not likely to be the result of general problems with saccadic control.
None of the groups differed in the time required to initiate reflexive saccades to visual targets, whether this was appropriate, in the case of the prosaccade task, or inappropriate, in the case of the antisaccade task. However, patients with acute schizophrenia and psychotic affective disorder tended to have longer latencies on correct antisaccade trials, in which voluntary saccades were made to imagined locations. Although the results are sometimes not statistically significant, almost all studies that divide reaction times on the antisaccade task into correct and incorrect trials report that schizophrenia patients have increased latencies only on correct trials (5, 14, 20, 32, 33). The fact that the schizophrenia patients had significantly increased latencies on correct antisaccade trials indicates that these patients had to compensate and slow their responses disproportionately in order to perform correctly.
In summary, the first-degree biological relatives of schizophrenia probands, who did not suffer from psychosis, showed marked difficulties suppressing unwanted saccades. We interpret this as indicating that the antisaccade task taps genetic risk for schizophrenia. In addition, regardless of clinical state, schizophrenia patients evidenced robust difficulties suppressing unwanted saccades during the antisaccade task. This effect was not related to possible confounds such as interfering psychotic symptoms, the effects of medication, or medication side effects. Indeed, schizophrenia patients whose illness was in full remission had marked difficulties suppressing unwanted saccades. Although the schizophrenia patients did show other signs of saccadic abnormalities, these problems did not account for the higher proportion of reflexive errors. Together, these results suggest that saccadic disinhibition, which may reflect prefrontal cortical abnormalities (6), is strongly associated with the genetic liability for schizophrenia.
 |
Received Oct. 19, 1999; revisions received April 3 and Aug. 8, 2000; accepted Aug. 10, 2000. From the Department of Psychology, University of Minnesota. Address reprint requests to Dr. Iacono, Department of Psychology, University of Minnesota, N218 Elliott Hall, 75 East River Rd., Minneapolis, MN 55455-0344; [email protected] (e-mail). Supported by NIMH grants MH-49738 and MH-17069 and an Eva O. Miller Fellowship (Dr. Curtis).
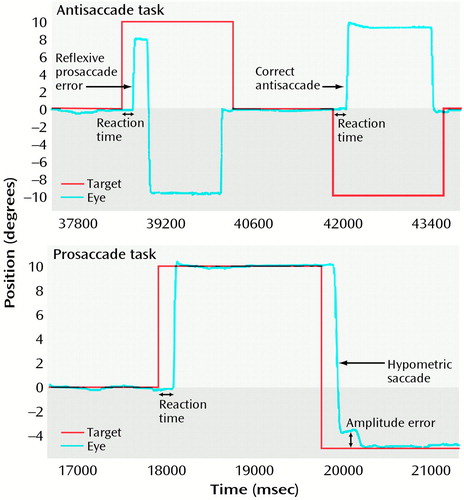
Figure 1. Measurement of Saccadic Performance of a Subject With Acute Schizophreniaa
aIn the antisaccade task, the subject makes a reflexive prosaccade error during the first trial in the direction of the cue that is followed by a corrective antisaccade toward the imagined correct location. On the second trial, the subject correctly inhibits making a saccade to the cue and generates an antisaccade to the imagined correct location. In the prosaccade task, the subject exhibits no amplitude error on the first visually guided saccade, whereas the second saccade is hypometric, i.e., its amplitude falls short of the visual target and requires a small corrective saccade.
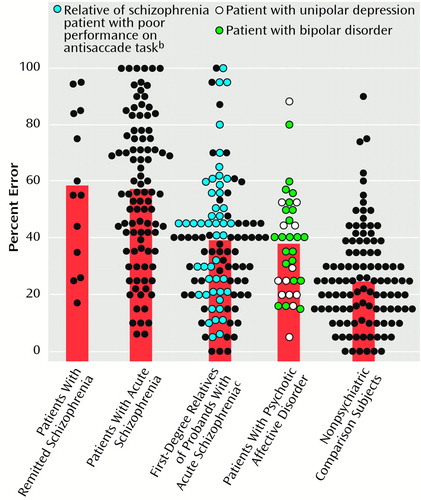
Figure 2. Antisaccade Performance of Patients With Remitted or Acute Schizophrenia, First-Degree Relatives of Probands With Acute Schizophrenia, Patients With Psychotic Affective Disorder, and Nonpsychiatric Comparison Subjectsa
aThe bars represent the group means. As determined by Student-Newman-Keuls follow-up tests that used an alpha level of 0.05, both schizophrenia groups made significantly more errors than the patients with psychotic affective disorder and the first-degree relatives, both of which groups, in turn, made significantly more errors than the nonpsychiatric comparison subjects. No other group differences were significant.
bPoor performance on the antisaccade task was defined as performance two standard deviations worse than that of the mean for the nonpsychiatric comparison subjects.
cRelatives with no lifetime psychotic diagnosis.
1. Hallett P: Primary and secondary saccades to goals defined by instructions. Vision Res 1978; 18:1279–1296Google Scholar
2. Clementz BA, McDowell JE, Zisook S: Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol 1994; 103:277–287Crossref, Medline, Google Scholar
3. Crawford TJ, Haeger B, Kennard C, Reveley MA, Henderson L: Saccadic abnormalities in psychotic patients, II: the role of neuroleptic treatment. Psychol Med 1995; 25:473–483Crossref, Medline, Google Scholar
4. Crawford TJ, Haeger B, Kennard C, Reveley MA, Henderson L: Saccadic abnormalities in psychotic patients, I: neuroleptic-free psychotic patients. Psychol Med 1995; 25:461–471Crossref, Medline, Google Scholar
5. Fukushima J, Fukushima K, Miyasaka K, Yamashita I: Voluntary control of saccadic eye movement in patients with frontal cortical lesions and parkinsonian patients in comparison with that in schizophrenics. Biol Psychiatry 1994; 36:21–30Crossref, Medline, Google Scholar
6. Curtis CE, Calkins ME, Iacono WG: Saccadic disinhibition in schizophrenia patients and their first-degree biological relatives: a parametric study of the effects of increasing inhibitory load. Exp Brain Res (in press)Google Scholar
7. Katsanis J, Kortenkamp S, Iacono WG, Grove WM: Antisaccade performance in patients with schizophrenia and affective disorder. J Abnorm Psychol 1997; 106:468–472Crossref, Medline, Google Scholar
8. Guitton D, Buchtel HA, Douglas RM: Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res 1985; 58:455–472Crossref, Medline, Google Scholar
9. Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch AI: Cortical control of saccades. Ann Neurol 1995; 37:557–567Crossref, Medline, Google Scholar
10. Crawford TJ, Puri BK, Nijran KS, Jones B, Kennard C, Lewis SW: Abnormal saccadic distractibility in patients with schizophrenia: a 99mTc-HMPAO SPET study. Psychol Med 1996; 26:265–277Crossref, Medline, Google Scholar
11. O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS: Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proc Natl Acad Sci USA 1995; 92:925–929Crossref, Medline, Google Scholar
12. Paus T, Petrides M, Evans AC, Meyer E: Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 1993; 70:453–469Crossref, Medline, Google Scholar
13. Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR: Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol 1996; 75:454–468Crossref, Medline, Google Scholar
14. McDowell JE, Clementz BA: The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Exp Brain Res 1997; 115:333–344Crossref, Medline, Google Scholar
15. McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA: Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology 1999; 36:138–141Crossref, Medline, Google Scholar
16. Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW: Saccadic eye movements in families multiply affected with schizophrenia: the Maudsley Family Study. Am J Psychiatry 1998; 155:1703–1710Google Scholar
17. Iacono WG: Identifying psychophysiological risk for psychopathology: examples from substance abuse and schizophrenia research. Psychophysiology 1998; 35:621–637Crossref, Medline, Google Scholar
18. Tien AY, Pearlson GD, Machlin SR, Bylsma FW, Hoehn-Saric R: Oculomotor performance in obsessive-compulsive disorder. Am J Psychiatry 1992; 149:641–646Link, Google Scholar
19. Rosenberg D, Averbach D, O’Hearn K, Seymour A, Birmaher B, Sweeney J: Oculomotor response inhibition abnormalities in pediatric obsessive-compulsive disorder. Arch Gen Psychiatry 1997; 54:831–838Crossref, Medline, Google Scholar
20. Sereno AB, Holzman PS: Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry 1995; 37:394–401Crossref, Medline, Google Scholar
21. Tien AY, Ross DE, Pearlson G, Strauss ME: Eye movements and psychopathology in schizophrenia and bipolar disorder. J Nerv Ment Dis 1996; 184:331–338Crossref, Medline, Google Scholar
22. Sweeney JA, Strojwas MH, Mann JJ, Thase ME: Prefrontal and cerebellar abnormalities in major depression: evidence from oculomotor studies. Biol Psychiatry 1998; 43:584–594Crossref, Medline, Google Scholar
23. Fukushima J, Fukushima K, Morita N, Yamashita I: Disturbances in the control of saccadic eye movement and eye-head coordination in schizophrenics. J Vestib Res 1990; 1:171–180Medline, Google Scholar
24. Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D: The Roscommon Family Study, I: methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry 1993; 50:527–540Crossref, Medline, Google Scholar
25. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
26. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1981Google Scholar
27. Barnes TR: A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154:672–676Crossref, Medline, Google Scholar
28. Simpson GM, Angus JW: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
29. Simpson GM, Lee JH, Zoubok B, Gardos G: A rating scale for tardive dyskinesia. Psychopharmacology (Berl) 1979; 64:171–179Crossref, Medline, Google Scholar
30. Myles-Worsley M, Coon H, McDowell J, Brenner C, Hoff M, Lind B, Bennett P, Freedman R, Clementz B, Byerley W: Linkage of a composite inhibitory phenotype to a chromosome 22q locus in eight Utah families. Am J Med Genet 1999; 88:544–550Crossref, Medline, Google Scholar
31. Thaker GK, Nguyen JA, Tamminga CA: Increased saccadic distractibility in tardive dyskinesia: functional evidence for subcortical GABA dysfunction. Biol Psychiatry 1989; 25:49–59Crossref, Medline, Google Scholar
32. Fukushima J, Fukushima K, Morita N, Yamashita I: Further analysis of the control of voluntary saccadic eye movements in schizophrenic patients. Biol Psychiatry 1990; 28:943–958Crossref, Medline, Google Scholar
33. Fukushima J, Morita N, Fukushima K, Chiba T, Tanaka S, Yamashita I: Voluntary control of saccadic eye movements in patients with schizophrenic and affective disorders. J Psychiatr Res 1990; 24:9–24Crossref, Medline, Google Scholar



