Childhood-Onset Psychotic Disorders: Magnetic Resonance Imaging of Volumetric Differences in Brain Structure
Abstract
OBJECTIVE: Although childhood-onset schizophrenia is rare, children with brief psychotic symptoms and prominent emotional disturbances commonly present diagnostic and treatment problems. Quantitative anatomic brain magnetic resonance images (MRIs) of a subgroup of children with psychotic disorder not otherwise specified were compared with those of children with childhood-onset schizophrenia and healthy comparison subjects. METHOD: Anatomic MRIs were obtained for 71 patients (44 with childhood-onset schizophrenia and 27 with psychotic disorder not otherwise specified) and 106 healthy volunteers. Most patients had been treated with neuroleptics. Volumetric measurements for the cerebrum, anterior frontal region, lateral ventricles, corpus callosum, caudate, putamen, globus pallidus, and midsagittal thalamic area were obtained. RESULTS: Patients had a smaller total cerebral volume than healthy comparison subjects. Analysis of covariance for total cerebral volume and age found that lateral ventricles were larger in both patient groups than in healthy comparison subjects and that schizophrenia patients had a smaller midsagittal thalamic area than both subjects with psychotic disorder not otherwise specified and healthy comparison subjects. CONCLUSIONS: Pediatric patients with psychotic disorder not otherwise specified showed a pattern of brain volumes similar to those found in childhood-onset schizophrenia. Neither group showed a decrease in volumes of temporal lobe structures. Prospective longitudinal magnetic resonance imaging and clinical follow-up studies of both groups are currently underway to further validate the distinction between these two disorders.
Across the branches of medicine, the study of individuals with the early onset of a multifactorial disease has been an important approach in decreasing the heterogeneity of study groups (1). Since 1990, a study of refractory childhood-onset schizophrenia (onset of psychotic symptoms by age 12) has been ongoing at the National Institute of Mental Health (NIMH) (2). Neurobiological studies suggest that childhood-onset schizophrenia is on a continuum with later-onset schizophrenia (3). The fact that childhood-onset schizophrenia is rare (4) and may be associated with higher rates of familial schizophrenia spectrum disorders (3) has led to the speculation that this form of the disorder may represent a particularly severe variant with a stronger genetic predisposition.
A sizable group of pediatric patients referred to the NIMH childhood-onset schizophrenia study who did not meet the DSM-III-R criteria for schizophrenia experienced brief hallucinations and delusions, typically under stress, that occurred a few times a month but revealed no evidence of thought disorder (5). Although these patients’ psychotic symptoms were ego-dystonic to patients and impaired patients’ functioning, these intermittent problems were less the focus of concern than were their dramatic mood outbursts and periodic aggression, which necessitated frequent psychiatric hospitalizations. The children in this cohort lacked the striking and pervasive disturbances in cognition, defective emotional rapport, bizarre hallucinations, and delusions that characterized the patients with childhood-onset schizophrenia. Although the distorted reality and affective lability of this group initially suggested borderline or schizotypal personality disorder, none of the children diagnosed with psychotic disorder not otherwise specified (DSM-IV, section 298.9) met the criteria for these disorders.
Children with atypical or subclinical presentations of schizophrenia are well recognized but have been difficult to classify (6–8). Because of the clinical heterogeneity of this group, a subgroup from the larger population of children with nonschizophrenic nonaffective psychoses, provisionally labeled “multidimensionally impaired” (9), has been studied at NIMH since 1990 (5). The rate of schizophrenia spectrum disorders in first-degree relatives appears elevated in this subgroup with psychotic disorder not otherwise specified, which is similar to what has been observed in childhood-onset schizophrenia (9). In addition to family history, this subgroup with psychotic disorder not otherwise specified shares a pattern of premorbid characteristics, cytogenetic abnormalities, and neuropsychological test impairments similar to those found in childhood-onset schizophrenia (9, 10). In the absence of diagnosis-specific biological markers for schizophrenia, the exact boundaries of childhood-onset schizophrenia remain unclear; the possibility exists that at least some of these cases of psychotic disorder not otherwise specified represent a phenotypic variant of childhood-onset schizophrenia.
To date, there have been no biological studies of children with atypical psychosis, in part because of the heterogeneity of this residual group. Evaluation of the brain structure in children with psychotic disorder not otherwise specified may offer insight into an endophenotype common to both that impairment and childhood-onset schizophrenia, whereas differences between the groups might implicate brain structures that contribute to the development of childhood-onset schizophrenia. Also, the ascertainment of a systematically defined group of children with psychotic disorder not otherwise specified may allow for more ambitious genetic studies examining the phenotypic features of childhood-onset psychotic disorders associated with the greatest familiality and heritability.
The present study examined the anatomic brain magnetic resonance imaging (MRI) characteristics of the first 27 children and adolescents with psychotic disorder not otherwise specified in the NIMH study to determine if their symptoms resembled those of a group of 44 patients with childhood-onset schizophrenia. Previous neuroimaging studies have indicated a possible decrease in total cerebral volume, a decrease in midsagittal thalamic area and cerebellar volumes, a larger total corpus callosum area, and significantly enlarged lateral ventricular volumes in patients with childhood-onset schizophrenia (11) that were similar to volumes noted in both adolescent- (12) and adult-onset schizophrenia (13). No significant diagnostic differences in temporal lobe or medial temporal lobe volumes were found on initial scans (3, 14), although at the 2- to 4-year follow-up a decrement was present in hippocampal volumes in patients with childhood-onset schizophrenia in relation to those of healthy comparison subjects (15).
On the basis of similarities in premorbid history, an elevated risk for schizophrenic spectrum disorders in first-degree relatives, cytogenetic abnormalities, and neuropsychological test impairments in patients with childhood-onset schizophrenia and patients with psychotic disorder not otherwise specified (9, 10), it was hypothesized that the group with psychotic disorder not otherwise specified would have a pattern of anatomic brain MRI abnormalities resembling those found in patients with childhood-onset schizophrenia.
Method
Subjects
Recruitment and diagnostic procedures have been described in detail elsewhere (9). In brief, girls and boys aged 6–18 who had been diagnosed with the DSM-III-R or DSM-IV criteria for schizophrenia were sought by means of national recruitment via professional and patient advocacy groups for an inpatient study involving treatment trials of atypical and typical neuroleptics. Inclusion criteria were 1) at least two previous unsuccessful neuroleptic trials because of either intolerable side effects or nonresponse, 2) an onset of psychotic symptoms by age 12 years, 3) a premorbid IQ greater than 70, and 4) the absence of significant medical or neurological disorders.
Of 950 referrals, more than 600 charts were reviewed, and 200 patients and their families were screened in person. Admission to the study followed a detailed screening, including a review of old medical charts and clinical and structured interviews with parents and child.
Patients With Psychotic Disorder Not Otherwise Specified
Thirty-two of the children exhibiting psychotic symptoms who did not meet the DSM-III-R criteria for schizophrenia were classified as having psychotic disorder not otherwise specified and given the provisional diagnostic label of “multidimensionally impaired disorder” by means of the following empirically derived criteria (5):
1. Poor ability to distinguish fantasy from reality as shown by ideas of reference and regular but brief perceptual disturbances during stress or while falling asleep.
2. Near-daily periods of emotional lability disproportionate to their precipitants.
3. Impaired interpersonal skills despite a desire to initiate social interactions with peers.
4. Multiple developmental cognitive deficits.
5. Absence of thought disorder.
On the basis of 71 consecutive in-person screening interviews of children referred to the NIMH study of childhood-onset schizophrenia, the interrater reliability for the subgroup with psychotic disorder not otherwise specified with the use of the five criteria was good (kappa=0.81) (5) and allowed the psychiatrists to distinguish this subgroup from children with overlapping clinical presentations such as bipolar disorder, attention deficit hyperactivity disorder (ADHD), and milder forms of autism.
Five children with psychotic disorder not otherwise specified did not participate for clinical reasons such as loss of residential placement or parental reluctance. Thus, MRI scans were obtained for 27 subjects with psychotic disorder not otherwise specified (23 boys and four girls). The adoption records of three (11%) of the 27 patients suggested that a biological parent had a diagnosis of schizophrenia, and eight (30%) of the patients had a first-degree relative with a diagnosis of schizotypal personality disorder on the basis of in-person structured diagnostic interviews performed at NIMH.
Socioeconomic status was generally middle class on the Hollingshead-Redlich Scale (mean=2.85, SD=1.06) (range=1 [high] to 5 [low]) (16). Parents were well educated. Nine (60%) of 15 fathers and 16 (70%) of 23 mothers available for in-person interview had at least partial college training. The mean full-scale IQ obtained at NIMH for the patients was 83.3 (SD=13.0). The mean standard score for the reading decoding subtest of the Kaufman Test of Educational Achievement (5, 17) obtained at NIMH for the 21 patients who were testable was 94.0 (SD=18.4). At the time of scanning, nine patients (33%) were receiving atypical neuroleptics, and 13 (48%) were receiving conventional neuroleptics.
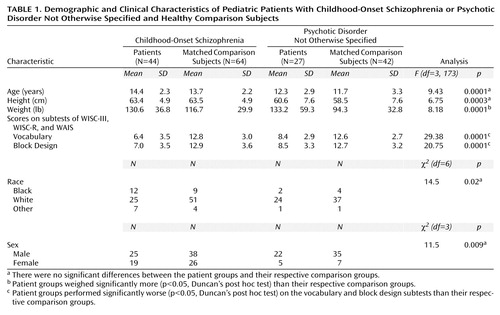
The demographic and clinical characteristics of 44 patients with childhood-onset schizophrenia are summarized in Table 1 and have been described in greater detail elsewhere (15).
Healthy Comparison Subjects
Healthy comparison subjects were selected from an ongoing study of brain development in healthy children and adolescents (18). Any physical or neurological disorder, lifetime history of any psychiatric disorder, or history of major psychiatric disorder in first-degree relatives was exclusionary. For this carefully screened group, approximately only one of six initial contacts was accepted for study inclusion. For this comparison, 42 healthy subjects were chosen for comparison to the group with psychotic disorder not otherwise specified, and 64 healthy subjects were chosen for comparison to the group with childhood-onset schizophrenia on the basis of age, sex, height, and handedness. The demographic and clinical characteristics of patients and healthy comparison subjects are summarized in Table 1.
The study was explained to the subjects and their parents. Written assent was obtained from each child, and written informed consent was obtained from the parents. The study protocol was approved by the institutional review board at NIMH.
MRI Procedures
MRI acquisition protocols, image analysis methods, and anatomical definitions for the measured brain regions are detailed elsewhere (11, 14, 18). In summary, subjects were scanned on a 1.5-T Signa scanner (General Electric, Milwaukee) located at the National Institutes of Health (NIH) Clinical Center in Bethesda, Md. A three-dimensional spoiled gradient recalled echo imaging sequence in the steady state (TE=5 msec, TR=24 msec, flip angle=45°, acquisition matrix=192 ≥ 256, number of excitations=1, field of view=24 cm) was used to obtain T1-weighted images. Two volumetric series with 1.5-mm thick axial and 2.0-mm thick coronal slices were acquired. The axial slice was used to quantify total cerebral volume and was reformatted for the midsagittal thalamic and corpus callosum measures. The coronal series was used for all other quantifications so as to sample the long axis of the structure or region. Head position was standardized by use of vitamin E capsules as external markers (one in the meatus of each ear and one taped to the left inferior orbital ridge, ensuring that all three were visible in the same axial reference plane). Rotation in the other plane was controlled by alignment of the nose to the 12:00 position. Head movement was minimized by placing foam padding on the sides of the subjects’ heads. Scans were performed in the evening to promote falling asleep in the scanner.
Image Analysis
The images were transferred to computer workstations, where they were analyzed by means of a variety of computer platforms and software packages, details of which have been presented elsewhere (18). Images were analyzed with raters blind to subject characteristics. Because motion artifacts caused differences in scan quality, not all structures were quantifiable for all subjects.
Total cerebral volume was quantified by means of a program that incorporates a priori knowledge of brain anatomy by modeling the brain as an active surface template, which then conforms to the individual brain through a series of energy minimization functions (19). Each axial slice of the output of this program was then edited by raters who removed artifacts related to eyeballs or patches of dura. The total cerebral volume measurement included ventricular CSF but excluded cerebellar and extracerebral CSF.
“Anterior frontal” was defined as brain matter anterior to a coronal plane intersecting the most anterior point of the corpus callosum. The orientation of the plane was standardized to be perpendicular to a line connecting the midline anterior and posterior commissures. Lateral ventricular volumes were calculated by summing area measurements from all coronal planes on which ventricles were visible by using an operator-supervised thresholding technique available on NIH’s Image 1.6 (20).
The caudate, putamen, globus pallidus, amygdala, hippocampus, and temporal lobes were manually outlined from coronal slices by means of Image 1.6 on a computer workstation. The caudate measure did not include the tail or the nucleus accumbens. The temporal stem was demarcated by a line connecting the most inferior point of the insular cisterns to the most lateral point of the basal cisterns above the hippocampus. The most posterior slice containing the splenium of the corpus callosum demarcated the posterior extent of the temporal lobes. The superior temporal gyrus was identified by its gyral boundary and traced throughout its extent. The most posterior slice containing fibers of the fornix was designated as the posterior boundary of this structure. The thalamus and corpus callosum area was quantified from a single midsagittal slice reconstructed from the axial series.
Anterior and posterior segments of the superior temporal gyrus were also identified, with the anterior segment ending at the most posterior slice before the appearance of the mamillary bodies. The slice was also designated as the posterior extent of the amygdala, while the first slice containing the mamillary bodies marked the anterior boundary of the hippocampus. The most posterior slice containing fibers of the fornix demarcated the posterior boundary of the hippocampus.
Ten brains were measured by two raters, revealing the following interrater reliabilities (intraclass correlation coefficient) for each structure: total cerebral volume=0.99, anterior frontal=0.97, caudate=0.88, putamen=0.84, globus pallidus=0.81, amygdala=0.86, hippocampus=0.87, temporal lobes=0.98, lateral ventricles=0.99, thalamus=0.85, and corpus callosum=0.92.
Data Analysis
Analyses were performed by means of SAS software (21). To examine overall group differences between patients and comparison subjects, all analyses of covariance (ANCOVAs) were used with all four groups (patients with childhood-onset schizophrenia, their matched healthy comparison subjects, patients with psychotic disorder not otherwise specified, and their matched healthy comparison subjects). Differences between patients and their respective healthy comparison subjects were determined with post hoc tests.
Because the patients had significantly smaller total cerebral volumes and because of the differences in age between the patient groups, ANCOVAs controlling for age and cerebral volume were used in one-way or two-way (group and side) repeated measures analysis with Duncan’s post hoc test. Comparisons were made on all demographic variables (sex, age, height, weight, handedness, and cognitive measures), as well as on the absolute measures of the brain regions of interest. All tests were two-tailed.
The power of this study to find a large effect size between patients and healthy comparison subjects was greater than 90%. The power to find a moderate effect size (0.4) between patients and healthy comparison subjects was approximately 65%.
Pearson’s product-moment correlations tested relationships between 1) patient MRI measures with values significantly different from those of comparison subjects and 2) clinical measures, including age, age at onset of psychotic symptoms, lifetime chlorpromazine-equivalent doses, full-scale IQ, premorbid impairments, obstetrical complications (22), and clinical ratings of psychiatric symptoms from the Scale for the Assessment of Negative Symptoms (SANS) (23) and the Scale for the Assessment of Positive Symptoms (SAPS) (24). Antipsychotic doses at the time of admission and total lifetime antipsychotic exposure (summed doses by length of treatment) were obtained by means of chart review and converted to lifetime chlorpromazine-equivalent doses for correlational analyses.
Results
Demographic and Clinical Characteristics
As shown in Table 1, an overall group difference was found between patients and their respective healthy comparison subjects on the WISC-III, WISC-R, and WAIS vocabulary and block design subtest scores and on subject weight. Post hoc testing indicated that patients performed significantly worse (p<0.05, Duncan’s post hoc test) on the vocabulary and block design subtests and weighed more (p<0.05, Duncan’s post hoc test) than their respective healthy comparison subjects. There were no overall group differences in age, height, and sex between the patients and their respective comparison groups.
Regional Brain Volumes
The patients had a generalized reduction in brain tissue, as shown by a total cerebral volume that was 4.2% smaller in patients with childhood-onset schizophrenia and 5.5% smaller in patients with psychotic disorder not otherwise specified than in their respective healthy comparison groups (Table 2). The remaining MRI brain volumes were analyzed with an ANCOVA for the entire study group by using total cerebral volume and age as covariates. There were significant main diagnostic effects for the lateral ventricle’s caudate, putamen, globus pallidus, and midsagittal thalamic area. Both lateral ventricular and basal ganglia volumes were increased in both groups of patients; however, the midsagittal thalamic area was only decreased in patients with childhood-onset schizophrenia compared to their respective healthy comparison subjects. There were no diagnostic differences for either group for the corpus callosum, anterior frontal, temporal lobe, hippocampus, superior temporal gyrus, and amygdala volumes. Mean unadjusted volumes of brain regions of interest are displayed in Table 2, along with the same measures adjusted for total cerebral volume and age. Table 3 shows the F and p values resulting from the analyses of variance (ANOVAs) and ANCOVAs.
Effects of Laterality
As shown in Table 3, no significant effects of laterality were noted for temporal lobe or medial-temporal lobe structures between patients and healthy comparison subjects. Significant interactions of side and diagnosis were demonstrated for the lateral ventricle’s caudate, putamen, and globus pallidus.
Although in healthy comparison subjects these structures were symmetric, post hoc testing found that there were significant asymmetries (p<0.05, Duncan’s post hoc test) for the lateral ventricles (left greater than right), putamen (left greater than right), caudate (right greater than left), and globus pallidus (right greater than left) in the two groups of patients.
Correlational Analyses
Clinical correlates were evaluated for all MRI volume measures that differed significantly from comparison values. No significant relationships were observed between IQ, age, age at onset of psychotic symptoms, premorbid adjustment, clinical ratings of psychotic symptoms from the SANS and SAPS, maternal obstetrical complications, lifetime chlorpromazine-equivalent doses, and lateral ventricular volumes or total cerebral volumes for either of the patient groups. Also, there were no significant correlations between lifetime chlorpromazine-equivalent doses and volumes of the globus pallidus or putamen.
Discussion
To our knowledge, this report represents the largest morphometric analysis of a systematically defined subgroup of children with narrowly defined childhood-onset schizophrenia and atypical psychosis. The major findings are that both the patients with childhood-onset schizophrenia and psychotic disorder not otherwise specified had significantly smaller total cerebral volumes and larger lateral ventricles than healthy comparison subjects.
The main purpose of this study was to investigate the pattern of structural brain MRI volumetric measures in a subgroup of children with psychotic disorder not otherwise specified who were labeled “multidimensionally impaired” and in children with schizophrenia. Children with psychotic disorder not otherwise specified share some phenomenological similarities to children with childhood-onset schizophrenia and appear to be at least as numerous as true patients with schizophrenia (25). They may be of importance for future biological and genetic studies of early-onset psychosis.
The subgroup with psychotic disorder not otherwise specified had a subtle reduction in total cerebral volume and an enlargement of the lateral ventricles that were similar to those volumes in patients with childhood-onset schizophrenia (3, 11) and patients with later-onset schizophrenia (26). It should be noted that although the majority of the patients with psychotic disorder not otherwise specified had comorbid ADHD and low average intellectual ability, lateral ventricular enlargement was not found in male patients with ADHD (27, 28) or mild mental retardation (29). Our findings suggest that the group with psychotic disorder not otherwise specified is similar to the group with childhood-onset schizophrenia with respect to brain morphology.
As reviewed by McCarley et al. (13), a significant proportion (75%) of MRI studies report volume differences in medial-temporal lobe structures in adult patients with schizophrenia, specifically reductions in the anterior portion of the amygdala-hippocampal complex, most notably on the left. Although we did not find volume reductions in the medial-temporal lobes in this subgroup of patients with psychotic disorder not otherwise specified, our patients with childhood-onset schizophrenia also showed no significant diagnostic differences in volume of the medial-temporal lobes at initial presentation. Similar findings have been reported for other patients with early-onset schizophrenia (30). Of interest, data for patients with childhood-onset schizophrenia suggest that structural brain abnormalities of the medial-temporal lobe may develop in adolescence (15).
Evidence of thalamic abnormalities in patients with childhood-onset schizophrenia (11) and patients with adult schizophrenia from neuroimaging and neuropathological studies have been well described (31–33). The thalamus serves as a major way station that appears to modulate input from a wide variety of brain regions and may be involved in filtering, gating, processing, and relaying information. However, the finding in this study of no thalamic volume differences for the group of patients with psychotic disorder not otherwise specified awaits replication with a valid bilateral volumetric measurement of this structure and thus should be interpreted conservatively.
It has been assumed that the generalized brain volume differences seen in patients with schizophrenia reflect the influence of factors contributing to disease susceptibility (34–36). However, the structural brain MRI abnormalities observed in this subgroup with psychotic disorder not otherwise specified may be nonspecific since a decrease in frontal lobe volume and an increase in ventricular volume has also been reported in a study of children hospitalized with depressive disorders (37). Our failure to identify the clinical correlates of the structural brain abnormalities found in patients with psychotic disorder not otherwise specified may reflect the small group size, the underlying complexity of the disorder, methodological problems involved in neuroimaging, and/or the effects of typical and atypical neuroleptic medication treatment.
It should be stressed that each patient with psychotic disorder not otherwise specified included in this study was referred to NIMH because a clinician believed the child to have schizophrenia. Although the cases of psychotic disorder not otherwise specified characterized in this study share some phenotypic features with children described as having “multiplex developmental disorder” (7, 8, 38), the symptoms of the latter construct appear to be closer to the autistic spectrum. Thus, we predict that children with multiplex developmental disorder would more closely resemble male subjects with autism in that both show enlargement of total brain tissue (39).
Finally, the subgroup with psychotic disorder not otherwise specified showed a significant enlargement of the caudate, globus pallidus, and putamen. Although the enlargement of these volumes did not show a significant quantitative relationship to previous neuroleptic exposure in patients with psychotic disorder not otherwise specified, a consistent pattern of association between exposure to typical neuroleptics and basal ganglia enlargement has been reported in patients with schizophrenia (40–42), and we have seen a reduction in basal ganglia volumes at 2-year follow-up in patients with childhood-onset schizophrenia when they were switched from treatment with typical to atypical neuroleptics (43). Functional imaging studies suggest that typical neuroleptic treatment is associated with changes in basal ganglia perfusion, and it has been hypothesized that a noticeable volume change could result from vascular engorgement resulting from greater perfusion and/or structural adaption to receptor blockade (40, 41).
The anatomic consequences of chronic antipsychotic exposure in humans is not well established. Glial proliferation and hypertrophy of the cerebral cortex has been reported in primates after 6 months of treatment with antipsychotic drugs (44). However, longitudinal MRI studies with parceling of brain regions are needed to assess the effects of typical and atypical neuroleptics on cortical anomalies in patients with schizophrenia. The differences in weight between patients and healthy comparison subjects most likely represent an effect of neuroleptic medication.
The study’s limitations should be considered in interpreting the results. There was a high variability of brain structures in both healthy comparison subjects and patients, making it difficult to detect and isolate small volume differences (18, 45). Thus, nonsignificant differences need to be interpreted cautiously.
Another limitation of this study is that the patients and healthy comparison subjects could not be matched for IQ. It was difficult to assess the effects of the marked differences in cognitive ability between the subgroup with psychotic disorder not otherwise specified and the group of healthy comparison subjects on the relatively modest changes in morphometric measures since in healthy children, IQ is positively correlated with total cerebral volume, in particular, with the prefrontal cortical gray volume (45). When the group differences in WISC-R vocabulary scores and age were taken into account, the finding of a reduction of total cerebral volume did not remain significant. However, the current IQ of the subgroup of patients with psychotic disorder not otherwise specified may not have been reflective of their true abilities (9), and/or it may not be an integral manifestation of the disorder.
In summary, children with psychotic disorder not otherwise specified appear to resemble children with childhood-onset schizophrenia more closely in terms of brain morphology than children with ADHD or other childhood neuropsychiatric disorders. Prospective clinical follow-up studies are underway to further validate this continuity.
 |
 |
 |
Received Sept. 24, 1999; revision received Feb. 25, 2000; accepted March 13, 2000. From the Child Psychiatry Branch, NIMH; the School of Medicine, Northwestern University, Chicago; and the School of Medicine, Yale University, New Haven, Conn. Address reprint requests to Dr. Rapoport, NIMH, Building 10, Room 3N202, 10 Center Drive MSC1600, Bethesda, MD 20892-1600; [email protected] (e-mail). The authors thank Drs. Jean Frazier and C.T. Gordon for their help with this study; Drs. F.X. Castellanos, M. Seeman, S. Frangou, and L. Ingraham for their suggestions; the NIMH clinical center staff, including Claudia Briguglio, R.N., M.N.; and the clinicians who referred patients to this study.
1. Childs B, Scriver CR: Age at onset and causes of disease. Perspect Biol Med 1986; 29:437–460Crossref, Medline, Google Scholar
2. McKenna K, Gordon CT, Rapoport JL: Childhood-onset schizophrenia: timely neurobiological research. J Am Acad Child Adolesc Psychiatry 1994; 33:771–781Crossref, Medline, Google Scholar
3. Nicolson R, Rapoport JL: Childhood onset schizophrenia: rare but worth studying. Biol Psychiatry 1999; 46:1418–1428Google Scholar
4. Beitchman JH: Childhood schizophrenia: a review and comparison with adult-onset schizophrenia. Psychiatr Clin North Am 1985; 8:793–814Crossref, Medline, Google Scholar
5. McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL: Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry 1994; 33:636–644Crossref, Medline, Google Scholar
6. Petti TA, Vela RM: Borderline disorders of childhood: an overview. J Am Acad Child Adolesc Psychiatry 1990; 29:327–337Crossref, Medline, Google Scholar
7. Towbin KE, Dykens EM, Pearson GS, Cohen DJ: Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry 1993; 32:775–782Crossref, Medline, Google Scholar
8. Cohen DJ, Towbin KE, Mayes L, Volkmar F: Developmental psychopathology of multiplex developmental disorder, in Developmental Follow-Up: Concepts, Domains, and Methods. Edited by Friedman SL, Haywood HC. Orlando, Fla, Academic Press, 1994, pp 155–179Google Scholar
9. Kumra S, Jacobsen LK, Lenane M, Zahn TP, Wiggs E, Alaghband-Rad J, Castellanos FX, Frazier JA, McKenna K, Gordon CT, Smith A, Hamburger SD, Rapoport JL: “Multidimensionally impaired disorder”: is it a variant of very early-onset schizophrenia? J Am Acad Child Adolesc Psychiatry 1998; 37:91–99Google Scholar
10. Kumra S, Wiggs E, Bedwell J, Smith AK, Arling E, Albus K, Hamburger SD, McKenna K, Jacobsen LK, Rapoport JL, Asarnow RF: Neuropsychological deficits in pediatric patients with childhood-onset schizophrenia and psychotic disorder not otherwise specified. Schizophr Res 2000; 42:135–144Crossref, Medline, Google Scholar
11. Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, Vaituzis AC, Rajapakse JG, Lenane MC, McKenna K, Jacobsen LK, Gordon CT, Breier A, Rapoport JL: Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry 1996; 53:617–624Crossref, Medline, Google Scholar
12. Frangou S, Kravariti J, Simmons A, Andrews C, Williams S, Pipe R, Murray R: The Maudsley Early Onset Schizophrenia Study: brain structural abnormalities in adolescent onset schizophrenia. Schizophr Res 1998; 29:77–78Crossref, Google Scholar
13. McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099–1119Google Scholar
14. Jacobsen LK, Giedd JN, Vaituzis AC, Hamburger SD, Rajapakse JC, Frazier JA, Kaysen D, Lenane MC, McKenna K, Gordon CT, Rapoport JL: Temporal lobe morphology in childhood-onset schizophrenia. Am J Psychiatry 1996; 153:355–361; correction, 153:851Link, Google Scholar
15. Giedd J, Jeffries NO, Blumenthal J, Castellanos FX, Vaituzis AC, Fernandez T, Hamburger SD, Liu H, Nelson J, Bedwell J, Tran L, Lenane M, Nicolson R, Rapoport JL: Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry 1999; 46:892–898Crossref, Medline, Google Scholar
16. Hollingshead AB, Redlich FC: Social Class and Mental Illness: A Community Study. New York, John Wiley & Sons, 1958Google Scholar
17. Kaufman AS, Kaufman NL: Kaufman Test of Educational Achievement. Circle Pines, Minn, American Guidance Service, 1985Google Scholar
18. Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL: Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex 1996; 6:551–560Crossref, Medline, Google Scholar
19. Snell JW, Merickel MB, Ortega JM, Goble JC, Brookeman JR, Kassell NF: Segmentation of the brain from 3D MRI using a hierarchical active surface template, in Proceedings of the SPIE: Medical Imaging 1994, vol 2167. Edited by Lowe MH. Bellingham, Wash, International Society for Optical Engineering, 1994, pp 2–9Google Scholar
20. Rasband W: Image 1.6. Bethesda, Md, National Institutes of Health, 1993Google Scholar
21. SAS Language and Procedures: Usage, version 6.07. Cary, NC, SAS Institute, 1989Google Scholar
22. Zax M, Sameroff AJ, Babigian HM: Birth outcomes in the offspring of mentally disordered women. Am J Orthopsychiatry 1977; 47:218–230Crossref, Medline, Google Scholar
23. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
24. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
25. Thomsen PH: Schizophrenia with childhood and adolescent onset: a nationwide register-based study. Acta Psychiatr Scand 1996; 94:187–193Crossref, Medline, Google Scholar
26. Raz S, Raz N: Structural brain abnormalities in the major psychoses: a quantitative review of the evidence from computerised imaging. Psychol Bull 1990; 108:93–108Crossref, Medline, Google Scholar
27. Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Rajapakse JC, Rapoport JL: Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1996; 53:607–616Crossref, Medline, Google Scholar
28. Filipek P, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy D, Biederman J: Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology 1997; 48:1–13Crossref, Google Scholar
29. Reiss AL, Abrams MT, Greenlaw R, Freund L, Denckla MB: Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat Med 1995; 1:159–167Crossref, Medline, Google Scholar
30. Marsh L, Harris D, Lim KO, Beal M, Hoff A, Minn K, Csernansky J, DeMent S, Faustman W, Sullivan EV, Pfefferbaum A: Structural magnetic resonance imaging abnormalities in men with severe chronic schizophrenia and an early age at clinical onset. Arch Gen Psychiatry 1997; 54:1104–1112Google Scholar
31. Pakkenberg B: The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr Res 1992; 7:95–100Crossref, Medline, Google Scholar
32. Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M: Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 1999; 46:908–920Crossref, Medline, Google Scholar
33. Hazlett EA, Buchsbaum MS, Byne W, Wei T-C, Spiegel-Cohen J, Geneve C, Kinderlehrer R, Haznedar MM, Shihabuddin L, Siever LJ: Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry 1999; 156:1190–1199Google Scholar
34. Walker E, Lewine RR, Neumann CS: Childhood behavioral characteristics and adult brain morphology in schizophrenia. Schizophr Res 1996; 22:93–101Crossref, Medline, Google Scholar
35. Lim KO, Beal M, Harvey R, Myers T, Lane B, Sullivan EV, Faustman W, Pfefferbaum A: Brain dysmorphology in adults with congenital rubella plus schizophrenia-like symptoms. Biol Psychiatry 1995; 37:764–776Crossref, Medline, Google Scholar
36. Woods BT, Yurgelun-Todd D, Goldstein JM, Seidman LJ, Tsuang MT: MRI brain abnormalities in chronic schizophrenia: one process or more? Biol Psychiatry 1996; 40:585–596Google Scholar
37. Steingard RJ, Renshaw PF, Yurgelun-Todd D, Appelmans KE, Lyoo IK, Shorrock KL, Bucci JP, Cesena M, Abebe D, Zurakowski D, Poussaint TY, Barnes P: Structural abnormalities in brain magnetic resonance images of depressed children. J Am Acad Child Adolesc Psychiatry 1996; 35:307–311Crossref, Medline, Google Scholar
38. Dahl EK, Cohen DJ, Provence S: Clinical and multivariate approaches to the nosology of pervasive developmental disorders. J Am Acad Child Psychiatry 1986; 25:170–180Crossref, Medline, Google Scholar
39. Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P: An MRI study of brain size in autism. Am J Psychiatry 1995; 152:1145–1149Google Scholar
40. Gur RE, Maany V, Mozley D, Swanson C, Bilker W, Gur RC: Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711–1717Google Scholar
41. Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC: Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry 1999; 156:1200–1204Google Scholar
42. Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M: Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine (letter). Lancet 1995; 345:456–457Crossref, Medline, Google Scholar
43. Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL: Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am J Psychiatry 1996; 153:564–566Link, Google Scholar
44. Selemon LD, Lidow MS, Goldman-Rakic PS: Increased volume and glial density in primate prefrontal cortex associated with chronic antipsychotic drug exposure. Biol Psychiatry 1999; 46:161–172Crossref, Medline, Google Scholar
45. Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB: Brain development, gender and IQ in children: a volumetric imaging study. Brain 1996; 119:1763–1774Google Scholar



