N-Acetylaspartate Concentration in the Anterior Cingulate of Maltreated Children and Adolescents With PTSD
Abstract
OBJECTIVE: Anterior cingulate dysfunction has been implicated in the pathophysiology of posttraumatic stress disorder (PTSD). The authors hypothesized that integrity of the anterior cingulate may be affected in childhood PTSD.METHOD: Single voxel proton magnetic resonance spectroscopy (proton MRS) was used to measure the relative concentration of N-acetylaspartate and creatine, a marker of neural integrity, in the anterior cingulate of 11 children and adolescents who met DSM-IV criteria for PTSD secondary to maltreatment and 11 healthy matched comparison subjects.RESULTS: The ratio of N-acetylaspartate to creatine was significantly lower in the maltreated subjects with PTSD than in the comparison subjects.CONCLUSIONS: The lower N-acetylaspartate/creatine ratio in subjects with PTSD suggests that anterior cingulate neuronal metabolism may be altered in childhood PTSD.
Symptoms of posttraumatic stress disorder (PTSD) represent pathological sequelae to traumatic experiences and may be thought of as conditioned fear responses to traumatic stimuli. The anterior cingulate cortex is involved in the extinction of conditioned fear responses and is implicated in the pathophysiology of PTSD (for review, see reference 1). Evidence for anterior cingulate dysfunction in adult PTSD comes from recent positron emission tomography studies. Studies comparing women who had been sexually abused as children and who had PTSD with women with a similar history who did not have PTSD found a lower level of anterior cingulate blood flow during traumatic script-driven imagery (2) and during memories of childhood sexual abuse (3). A lower level of anterior cingulate blood flow has also been seen in Vietnam combat veterans with PTSD compared to those without PTSD during exposure to combat-related traumatic stimuli (4).
Maltreatment of children is a cause of PTSD in children and adolescents (5) and may be associated with brain structural alterations that can be observed by using magnetic resonance imaging (6). Few studies have examined the in vivo neurochemistry of such neurobiological alterations. Magnetic resonance spectroscopy (MRS) is a safe means to measure neurobiological alterations in the brains of living children. The N-acetyl signal in the proton (1H) spectrum mainly comprises N-acetylaspartate and a small proportion of N-acetylaspartylglutamate. The total N-acetylaspartate signal is considered to be a marker of neural integrity. Decreased N-acetylaspartate concentrations are associated with increased metabolism and loss of neurons (for review, see reference 7).
In this pilot study, we hypothesized that proton MRS may identify altered neuronal integrity in the anterior cingulate in childhood PTSD. Specifically, we predicted that N-acetylaspartate would be reduced in the anterior cingulate in maltreated children and adolescents with PTSD compared to matched healthy comparison subjects.
Method
Eleven subjects who met DSM-IV criteria for PTSD secondary to maltreatment and 11 healthy comparison subjects were recruited for single voxel proton MRS studies of the anterior cingulate region. Subjects were case matched within 6 months of age (mean age=10.23 years [SD=2.8] for the PTSD group and mean=10.15 years [SD=3.0] for the comparison group; age range=4.2–14.8 years) and by sex (six male pairs and five female pairs), race, Tanner stage, and Hollingshead four-factor index of socioeconomic status (8) (mean=38.0 [SD=10.0] for the PTSD group and mean=38.4 [SD=7.5] for the comparison group). Subjects were case matched within 8 points of full-scale IQ (mean=114.9 [SD=12.5] for the PTSD group and mean=119.9 [SD=10.3] for the comparison group). Subjects were similar in weight (mean=85.5 lb [SD=31.9] for the PTSD group and mean=93.2 lb [SD=37.5] for the comparison group), height (mean=54.0 inches [SD=7.3] for the PTSD group and mean=55.7 inches [SD=8.0] for the comparison group), and intracranial volume (mean=1449.1 cm3 [SD=142.1] for the PTSD group and mean=1487.1 cm3 [SD=175.0] for the comparison group). Axis I mental disorders were assessed by using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version, which includes a comprehensive PTSD interview (9). Comorbidity in the PTSD group included: major depressive disorder (N=6), oppositional defiant disorder (N=5), and attention deficit hyperactivity disorder (N=3). PTSD traumas included sexual abuse (N=7), physical abuse (N=2), and witnessing domestic violence (N=2). An estimate of subjects’ IQ was made on the basis of the vocabulary, digit span, block design, and object assembly subscales of the Wechsler Intelligence Scale for Children (10). An assessment of handedness was made by using the Revised Physical and Neurological Examination for Subtle Signs inventory (11); subjects with a rating of right-handedness on eight of the inventory’s 12 handedness items were considered right-handed. All subjects except two in the PTSD group were right-handed. Exclusion criteria were 1) lifetime prior exposure to psychotropic drugs, 2) a significant medical or neurological illness or history of head injury, 3) gross obesity or growth failure, 4) full-scale IQ lower than 80, 5) presence of DSM-IV anorexia nervosa, autism, substance use disorder, or schizophrenia, and 6) a history of maltreatment or axis I disorder in comparison subjects. After a complete description of the study was given to subjects and their parents, written informed consent was obtained. Subjects received monetary compensation for participation.
For each subject, we obtained a single voxel 1H spectrum using a short TE stimulated echo acquisition sequence placed in the medial prefrontal cortex with the voxel centered on the anterior cingulate. We used a GE 1.5 Tesla scanner (Signa System, General Electric Medical Systems, Milwaukee) running version 5.4 software located at the Magnetic Resonance Research Center at the University of Pittsburgh Medical Center. The position of the voxel (2 ≥1.5 ≥1 cm, volume=3 cc) was determined from sagittal and coronal MR images at the anterior-most point of the genu of the corpus callosum, as previously described (12). The short TE stimulated echo acquisition sequence is part of the GE spectroscopy package and utilizes the 90E-90E-90E stimulated echo sequence (13). A TR of 1.5 seconds and 150 acquisitions was used. The MR spectrum was obtained with maximal receiver gain, 2000 Hz sweep width, and 2048 point spectral resolution. Spectral signal assignments were performed for N-acetylaspartate, choline, and creatine by using estimations from an external standard, i.e., from spectra of phantoms containing known concentrations of these compounds using the LCModel software package (licensed by S.W. Provencher, Max-Planck-Institut, Gottingen, Germany). The LCModel is a user-independent fitting routine that employs a library of concentration-calibrated model spectra of all individual metabolites as an a priori database for assigning MRS signals to their appropriate frequencies. Thus, this program enables quantification of metabolites with overlapping signals. It has been demonstrated to be an accurate and reliable method for quantifying short TE 1H spectrum MRS data (13, 14).
Because creatine is thought to be a measure of overall brain metabolism and is generally stable (for review, see reference 15), total N-acetylaspartate was quantified in relationship to creatine. In view of the very close matching of the PTSD and comparison subjects, we used two-tailed paired t tests for group comparisons (16).
Results
The total N-acetylaspartate/creatine ratio was lower in the subjects with PTSD than in the matched comparison subjects (mean=1.19 [SD=0.28] versus mean=1.35 [SD=0.23] in the comparison group; mean difference=0.15 [SD=0.23]) (paired t=2.24, df=10, p<0.05) (Figure 1). The choline/creatine ratio did not differ between groups (mean=0.22 [SD=0.06] for the PTSD group versus mean=0.23, [SD=0.04] for the comparison group) (paired t=0.45, df=10, p=0.66).
Discussion
To our knowledge, this is the first report of lower levels of N-acetylaspartate in the anterior cingulate region in maltreated children and adolescents with PTSD, compared with healthy matched subjects. The lower N-acetylaspartate/creatine ratios are suggestive of neuronal loss in the anterior cingulate. These findings may reflect global neuronal loss in childhood PTSD, a possibility supported by our previous findings that maltreated subjects with PTSD have smaller intracranial and cerebral volumes, smaller corpus callosal size, and larger lateral ventricles than comparison subjects (6). In our previous work, we also found a trend for smaller size of the genu, an area of the corpus callosum whose axons subserve the anterior cingulate region. Taken together, the results of our earlier work (6, 17) and of the pilot study reported here suggest that neuronal loss in childhood PTSD may contribute to the pathogenesis of PTSD and other impairments in psychosocial and cognitive functioning that are commonly seen in maltreated children with PTSD (18).
The study reported here must be considered preliminary because of the following limitations: small sample size, the use of a single voxel for MRS studies, tissue heterogeneity within the voxel, and lack of absolute quantification in our approach to metabolite measurements. We also note that this study is cross sectional, and our findings do not imply causation. If the findings are replicated, they may aid in understanding the pathophysiology of PTSD in maltreated children.
Received Oct. 8, 1999; revision received Jan. 6, 2000; accepted Jan. 12, 2000. From the Department of Psychiatry, University of Pittsburgh Medical Center; and Western Psychiatric Institute and Clinic, Pittsburgh. Address reprint requests to Dr. De Bellis, Developmental Traumatology Neuroimaging Laboratory, Western Psychiatric Institute and Clinic, 3811 O’Hara St., Pittsburgh, PA 15213; DeBellisMD@ msx.upmc.edu (e-mail). Supported in part by NIMH grants MH-01324 (Dr. De Bellis) and MH-01180 and MH-43687 (Dr. Keshavan). The authors thank Grace Moritz, M.S.W., Cara Renzelli, M.S.Ed., Sue R. Beers, Ph.D., and Neal D. Ryan, M.D., for their assistance in this work.
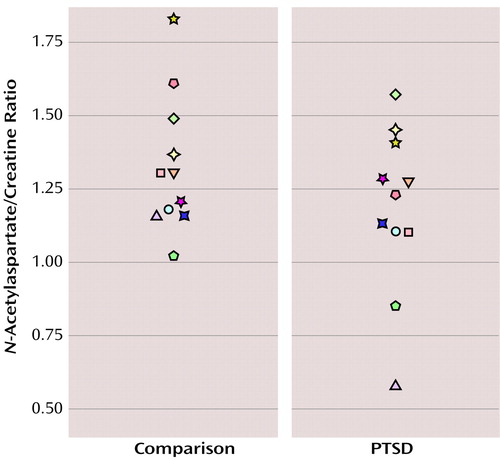
Figure 1. Anterior Cingulate N-Acetylaspartate/Creatine Ratios in 11 Maltreated Children and Adolescents With PTSD and 11 Healthy Matched Comparison Subjectsa
aPaired t=2.24, df=10, p<0.05.
1. Hamner MB, Lorberbaum JP, George MS: Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depress Anxiety 1999; 9:1–14Crossref, Medline, Google Scholar
2. Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK: Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry 1999; 156:575–584Abstract, Google Scholar
3. Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS: Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 1999; 156:1787–1795Google Scholar
4. Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS: Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry 1999; 45:806–816Crossref, Medline, Google Scholar
5. De Bellis MD: Posttraumatic stress disorder and acute stress disorder, in Handbook of Prevention and Treatment With Children and Adolescents. Edited by Ammerman RT, Hersen M. New York, John Wiley & Sons, 1997, pp 455–494Google Scholar
6. De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND: AE Bennett Research Award: developmental traumatology, part II: brain development. Biol Psychiatry 1999; 45:1271–1284Google Scholar
7. Prichard JW: MRS of the brain-prospects for clinical application, in MR Spectroscopy: Clinical Applications and Techniques. Edited by Young IR, Charles HC. London, Livery House, 1996, pp 1–25Google Scholar
8. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
9. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
10. Wechsler D: Manual for the Wechsler Intelligence Scale for Children—Revised. New York, Psychological Corp, 1974Google Scholar
11. Denckla MB: Revised Physical and Neurological Examination for Soft Signs. Psychopharmacol Bull 1985; 21:773–800Medline, Google Scholar
12. Keshavan MS, Montrose DM, Pierri JN, Dick EL, Rosenberg D, Talagala L, Sweeney JA: Magnetic resonance imaging and spectroscopy in offspring at risk for schizophrenia: preliminary studies. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21:1285–1295Google Scholar
13. Frahm J, Hanefeld F: Localized proton magnetic resonance spectroscopy of cerebral metabolites. Neuropediatrics 1996; 27:64–69Crossref, Medline, Google Scholar
14. Provencher SW: Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30:672–679Crossref, Medline, Google Scholar
15. Keshavan MS, Kapur S, Pettegrew JW: Magnetic resonance spectroscopy in psychiatry: potential, pitfalls, and promise. Am J Psychiatry 1991; 148:976–985Link, Google Scholar
16. Kirk R: Experimental Design: Procedures for the Behavioral Sciences. New York, Brooks/Coles, 1968Google Scholar
17. De Bellis MD, Baum A, Birmaher B, Keshavan M, Eccard CH, Boring AM, Jenkins FJ, Ryan ND: AE Bennett Research Award: developmental traumatology: part I: biological stress systems. Biol Psychiatry 1999; 45:1259–1270Google Scholar
18. De Bellis MD, Putnam FW: The psychobiology of childhood maltreatment. Child Adolesc Psychiatr Clin N Am 1994; 3:663–677Crossref, Google Scholar



