Continuous Performance Test and Schizophrenia: A Test of Stimulus-Response Compatibility, Working Memory, Response Readiness, or None of the Above?
Abstract
OBJECTIVE: Abnormalities of attention are considered the fundamental deficits in cognitive function manifested by patients with schizophrenia. The authors administered variations of two types of cognitive tasks to patients with schizophrenia (N=20) and normal comparison subjects (N=30) to test four possible cognitive mechanisms that might account for such abnormalities. METHOD: Variations of the Continuous Performance Test were used to test the four mechanisms. Stimulus-response mapping was explored by comparing results on a task in which subjects were to make a response if the word “nine” was preceded by the word “one” with results on a task in which the required response was made explicit by the stimulus (the word “ready” followed by the word “press”). The building up of a prepotent response tendency was tested by manipulating the probability with which the cue and imperative stimulus appeared (17% or 50%). The amount of working memory required to maintain contextual information was tested by using different delay intervals (1000 msec and 3000 msec). The extent to which problems in vigilance might be attributable to problems in the “motoric” component of response readiness was operationalized by having subjects perform a secondary motor task concurrent with the attentional task. RESULTS: Patients with schizophrenia performed significantly worse than the normal comparison subjects on all tasks. However, none of the four manipulations of the Continuous Performance Test tasks had a differential impact on the patients’ performance speed or accuracy. In contrast, there was a significant interaction of group, delay interval, and target probability in which patients made disproportionately more omission errors at short delay intervals and at low target probabilities. CONCLUSIONS: The findings may call into question the explanatory power of certain well-known contemporary mechanistic accounts of performance on the Continuous Performance Test in patients with schizophrenia. The findings suggest that a difficulty in rapidly encoding information (i.e., constructing a representation) in certain “unengaging” situations may be at the core of deficits on tasks associated with this attentional test.
The issue of sustained, focused attention has been prominent in many clinical and experimental descriptions of impairments in performance among patients with schizophrenia. Shakow’s work (1), in particular, maintained that the central cognitive dysfunction in schizophrenia was an inability to maintain a major task set; in other words, the problem was one of sustaining readiness to respond to task-relevant or signal stimuli over a period of time.
The Continuous Performance Test (2) has been widely used to measure sustained attentional deficits in schizophrenia. The test measures visual vigilance, which has been used as a principal experimental index of these deficits. In the basic form of this test, subjects monitor a random series of single numbers or letters, which are presented continuously, often at a rate of approximately one per second. Subjects are asked to indicate that they have detected a target event by pressing a response button and to avoid responding to distracting stimuli. The target event may be the appearance of a single stimulus (e.g., the letter “X” appearing in a sequence of letters; this task can be termed Continuous Performance Test–Single) or a stimulus appearing in a particular context (e.g., the letter “X” only when it follows the letter “A;” this task has been termed the Continuous Performance Test–AX) (2). Subsequent modifications have been made to increase sensitivity (e.g., through degradation of stimuli or use of two consecutive identical stimuli in which the target event is the second stimulus [Continuous Performance Test, Identical Pairs Version]) (3). Several hypotheses concerned with performance of patients with schizophrenia on the Continuous Performance Test have been proposed. For example, the finding that patients with schizophrenia perform especially poorly on vigilance tasks with high processing loads has led to suggestions that the deficiencies are due to a “reduction in available processing capacity or a temporary disruption of automatic as well as attention-demanding processes” (4). Patients’ attentional deficits seem to be most reliably elicited on variants of the Continuous Performance Test that have relatively high processing loads (i.e., tests with rapid stimulus presentation, those with stimulus degradation, or those in which memory of the previous stimulus is necessary to make the current decision). Thus the essential problem would seem to be a difficulty with the high moment-to-moment demand on processing capacity. Although the essential components of this deficit remain obscure, Cohen and colleagues (5, 6) recently operationalized a possible mechanism for this deficit. By adopting a computational approach, they proposed a defective mechanism that would become evident in performance on the Continuous Performance Test when the subject must keep the stimulus information in mind during a delay period between one stimulus and the next. This requirement would tax the already limited available processing capacity, with the result of poor performance in patients with schizophrenia. In the study reported here we wished to explore this issue further by empirically evaluating four possible mechanistic accounts of performance on the Continuous Performance Test in patients with schizophrenia.
Background
The four possible mechanisms involved in the performance of patients with schizophrenia on the Continuous Performance Test are discussed below.
Stimulus-Response Mapping
The first issue concerned the amount of stimulus-response mapping that is necessary when a stimulus is seen and a response specified by the experimenter is to be made. For example, in the “one-nine” version of the Continuous Performance Test, subjects are told to make a response when “nine” appears if it was preceded by “one.” The stimuli “one” and “nine” do not in themselves contain the necessary information required to make the specified response. Thus, the subject has to remember the instructions given by the experimenter in order to transform the stimulus (“nine”) into a response (“nine” means press the response button) (see reference 7). We manipulated the number of stages involved in this stimulus-response mapping process to see whether problems in this cognitive mechanism were responsible for the disproportionately worse performance on the Continuous Performance Test that is typically observed in patients with schizophrenia. Two kinds of stimuli were used: 1) cues and imperative stimuli consisting of numbers written as words, such as “one” (to signal the subject to get ready), “nine” (to signal the subject to press the response key), and “five” (to signal the subject to refrain from pressing a key); and 2) stimuli with inherent instructions on how to perform the task, such as “ready” (to signal the subject to get ready), “press” (to signal the subject to press the response key), and “no” (to signal the subject to refrain from pressing any key). We predicted that if the amount of stimulus-response mapping necessary in a Continuous Performance Test is responsible for the worse performance of patients with schizophrenia, a disproportionate deficit in patients would be especially evident in the “one-nine” version of the test but would be absent in the “ready-press” version.
The next two issues (target probability and delay interval) were based on the computational model of the core cognitive deficit in schizophrenia that was developed by Cohen and Servan-Schreiber (5). They hypothesized that several deficits in schizophrenia reflect a common underlying problem in maintaining and using “the internal representation of context to control action”; context was defined as “information held in mind in such a form that it can be used to mediate an appropriate behavioural response” (p. 46). Specifically, context is necessary to overcome a prepotent but task-inappropriate response. They suggested that by introducing asymmetry in response strength into the Continuous Performance Test by varying the frequency with which target stimuli appear, a test will be produced that is more sensitive to context problems in patients with schizophrenia (see also reference 6). It is to this variable that we now turn.
Target Probability
The second variable concerned building up a prepotent response tendency to make the test differentially sensitive to attentional problems in schizophrenia; the aim was to elicit problems in patients when they had to inhibit contextually inappropriate responses. We compared performance on tasks in which the cue and imperative stimulus (e.g., “one” and “nine” or “ready” and “press”) appeared together with either 17% probability or 50% probability. We predicted that patients with schizophrenia would make disproportionately more errors (commission errors) in a target-rich environment (i.e., 50% target probability) by responding to stimuli that were not preceded by appropriate cues; we hypothesized that these errors would be due to patients’ problems in overriding the prepotent response tendency.
Delay Intervals
The third variable we explored was the amount of working memory required to maintain the contextual information for different delay intervals. This variable was also based on the work of Cohen and Servan-Schreiber (5), who proposed that the representation of context is necessary to hold information about the stimulus in mind during the delay between one stimulus and the next. They argued that increasing the delay should render the test more sensitive to problems in the internal representation of context.
We compared performance on tasks with two different intervals between the onset of one stimulus and the onset of the next stimulus: 1000 msec and 3000 msec. (The intervals are referred to as stimulus onset asynchronies [SOAs].) The subject had to hold the task context in working memory to respond to the imperative stimulus appropriately. If this component is a key factor in optimal performance on the Continuous Performance Test, we predicted that long SOAs should be especially detrimental to the performance of patients with schizophrenia. However, patients’ performance on the “ready-press” task, where the task instructions are evident in the stimuli themselves, would not be reduced. Indeed, previous studies with patients with schizophrenia have shown that increasing the SOA from 1000 msec to 2000 or 5000 msec resulted in a marked decline in accuracy with longer delay periods (5, 6, 8, unpublished 1998 manuscript of Elvevåg).
Response Readiness
The fourth variable of interest was the extent to which problems in vigilance might be attributable to problems in the motor component of response readiness. We explored this variable by having subjects perform a second task concurrently with the attentional task, i.e., in a dual-task paradigm. We reasoned that if certain attentional problems revealed on the Continuous Performance Test reflect a problem with response readiness, i.e., the ability to load the motor programs in the motor system that allow one to execute a response (see, for example, reference 9), then the concurrent motor task would disproportionately worsen performance on the Continuous Performance Test in patients with schizophrenia. This explanation could account for certain clinical phenomena, such as patients’ noting that they should have pressed a button in response to a target, suggesting that they attended to the stimuli but somehow were not prepared motorically to respond.
Experiment 1
Method
Baseline tests
Two baseline tests were used to provide an index measure of subjects’ intellectual functioning. The first, the Wide Range Achievement Test, Revised (WRAT-R) (10), is widely used as an estimate of premorbid functioning. The second, the Wechsler Adult Intelligence Scale—Revised (WAIS-R) (11), is used to estimate current functioning. A short form of the WAIS-R was used.
Subjects
Twenty inpatients (18 male and two female patients) from the National Institute of Mental Health Neuropsychiatric Research Hospital participated in this study. All patients fulfilled DSM-IV criteria for schizophrenia as determined by the Structured Clinical Interview for DSM-IV (SCID) (12). The mean age of patients was 35.7 years (SD=9.17, range=19–56), and the mean premorbid IQ was 93.3 (SD=14.18, range=59–118), as measured by the WRAT-R, and was 90.8 (SD=12.86, range=68–111), as measured by the WAIS-R. Patients generally had multiple hospital admissions due to incomplete responses to conventional treatments. All of the patients were receiving neuroleptic medication at the time of the study. Six received clozapine or olanzapine, eight received risperidone, and six received high-potency antipsychotic drugs such as haloperidol, fluphenazine, or loxapine. One patient received both olanzapine and a high-potency neuroleptic (prolixin). Seven patients also received anticholinergic medication, and eight received other adjunctive medication. The comparison group comprised 30 normal healthy volunteers (15 male and 15 female subjects) who were recruited through the National Institutes of Health volunteer panel. The mean age of comparison subjects was 29.7 years (SD=7.37, range=21–50), and their mean IQ was 108.8 (SD=11.48, range=81–121), as measured by the WRAT-R, and was 110.7 (SD=12.35, range=81–127), as measured by the WAIS-R. The two groups differed significantly in age (t=2.50, df=47, p<0.05) and in mean scores on the WRAT-R (t=–3.93, df=40, p<0.001) and the WAIS-R (t=–5.17, df=41, p<0.0001). Subjects with a history of traumatic brain injury, epilepsy, developmental disorder, diagnosable substance dependence, or other known neurologic condition were excluded from the study. All subjects had normal or corrected-to-normal vision. All comparison subjects were paid for their participation. Patients completed the study as part of their protocol for entering the hospital and after their written informed consent had been obtained.
Apparatus
Stimuli were displayed on a computer monitor. Stimulus generation, reaction time measurement, and data recording were all controlled by an Apple Macintosh IIci computer that was running Cedrus Superlab software.
Design
There were three experimental variables: 1) two types of stimuli (“one-nine” or “ready-press”), 2) two target probabilities (17% and 50%), and 3) two SOAs (1000 and 3000 msec). Thus, when one of the two possible conditions of each variable was combined with the others, there were eight tasks in total.
The two types of stimuli were the words “zero,” “one,” “two,” “three,” “four,” “five,” “six,” “seven,” “eight,” and “nine” (the “one-nine” task) or the words “ready?,” “press,” and “no” (the “ready-press” task). The words were formed with a standard lowercase Times New Roman size 36 font in bold and were centered on the screen. Subjects were seated opposite the display screen at a viewing distance of approximately 50 cm.
The two target probabilities were 17% and 50%. Where the target probability was 17%, the subject was to make a response 15 times (i.e., respond to 30 of 180 words). Where the target probability was 50%, the subject was to respond 45 times (i.e., to 90 of 180 words). We found it difficult to increase the target probability much more without producing an obvious and predictable pattern (e.g., one, nine, four, one, nine, six, one, nine, etc.).
There were two SOAs: 1000 msec and 3000 msec.
Procedure
The order of the tasks was constant across all subjects. The Continuous Performance Tests were interspersed among other neuropsychological tests. Before subjects began any task, they were given practice trials. Each task comprised 180 stimuli in which the words “zero” to “nine” were presented. Each stimulus was displayed for 200 msec, followed by a blank interval of 800 or 2800 msec. Then the next trial commenced. The stimulus type, target probability, and SOA were always consistent within each task of 180 stimuli. The order of stimuli (e.g., “zero,” “five,” “nine,” etc.) throughout the 180 trials of the task was random but constant across subjects. (The sequence of words in the task with 17% target probability matched the sequence of digits in the “1-9” [digit] task in the Gordon Systems [GDS-Model II] [13].) Subjects were to make responses by pressing a computer key with their right index finger. The computer program was designed to measure reaction time in milliseconds from the onset of the imperative stimulus to the subject’s key response.
Results
Three types of data were analyzed: 1) reaction times, 2) omission errors, and 3) commission errors. All data were all submitted to analysis of variance (ANOVA), with subject group (patients and comparison subjects) as the between-group factor. Within-group factors were task type (“one-nine,” “ready-press”), target probability (17%, 50%), and SOA (1000 msec, 3000 msec). Although the data in Figure 1, Figure 2, and Figure 3 represent the untransformed results, data on omission and commission errors were transformed before the analyses because of potential floor effects. An empirical log odds transformation was used (14). For data on reaction time, in the few cases where cells were empty, the pooled reaction time for that group was calculated and inserted into the empty cell.
Reaction times
As expected, patients with schizophrenia had slower reaction times than comparison subjects (F=5.30, df=1, 48, p<0.05) (Figure 1). Further, there was a main effect of task type (F=16.84, df=1, 48, p<0.001), such that response latencies were faster on the “ready-press” task; a main effect of target probability (F=21.93, df=1, 48, p<0.0001), in which reaction times were faster for tasks with 50% target probability; and a main effect of SOA (F=75.62, df=1, 48, p<0.0001), in which responses were faster for tasks where the SOA was 1000 msec. (Increases in reaction time with an increasing foreperiod between an uninformative cue and an imperative stimulus are often reported and frequently ascribed to temporal uncertainty [15].) There was a significant two-way interaction of task type and target probability (F=4.59, df=1, 48, p<0.05). This interaction was important because it indicated that patients had differential impairments when SOAs were short and target probability was low. This result is consistent with the idea of an encoding deficit in patients with schizophrenia. There were no other significant interactions (Table 1). In particular, patients’ performance was not differentially improved in the “ready-press” condition.
Omission errors
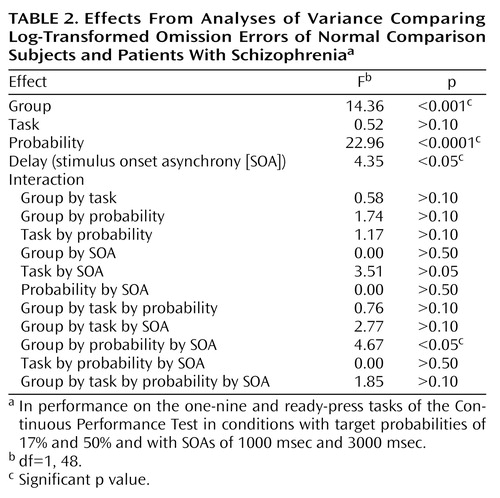
As expected, patients made significantly more omission errors than comparison subjects (F=14.36, df=1, 48, p<0.001). In addition, there was a main effect of target probability (F=22.96, df=1, 48, p<0.0001), in which more omissions were made when the target probability was 50%, and a main effect of SOA (F=4.35, df=1, 48, p<0.05), in which more omissions were made when the SOA was only 1000 msec (Figure 2). No significant main effect was found for task type (F=0.52, df=1, 48, p>0.10), perhaps because of ceiling effects in the comparison subjects. There was a significant three-way interaction between group, target probability, and SOA (F=4.67, df=1, 48, p<0.05), due to patients’ relatively worse performance in the low target probability condition when the SOA was brief. There were no other significant interactions (Table 2). In particular, patients’ performance was not differentially improved.
Commission errors
Patients made a disproportionate number of commission errors, although the difference between groups was marginal (F=3.79, df=1, 48, p=0.057). There was a significant main effect of target probability (F=28.09, df=1, 48, p<0.0001) (Figure 3), because all subjects had the chance to make more commission errors on the 50% target-probability task. There were no significant main effects of task type (F=0.03, df=1, 48, p>0.50) or SOA (F=1.89, df=1, 48, p>0.10), and no significant interactions (Table 3).
Experiment 2
Next we wanted to explore the extent to which response readiness, i.e., ability to load motor programs to allow one to make a response, contributes to performance on the Continuous Performance Test. We hypothesized that a concurrent motor task (tapping a key on a computer keyboard) would disproportionately worsen performance on the cognitive tasks in patients with schizophrenia.
Method
Baseline tests, subjects, and apparatus
Baseline tests and subjects were as described for experiment 1. The apparatus was the same as that used in experiment 1, except that an additional computer was used and subjects were asked to tap a key on the keyboard as fast as they could. The number of taps was recorded by the computer.
Design
Two of the Continuous Performance Tests used in experiment 1 were also used here (the “ready-press” task with an SOA of 1000 msec and with a target probability of 17% and the same task with the same SOA but with a target probability of 50%). In this experiment, subjects responded to the Continuous Performance Tests with the right index finger at the same time they were tapping with the left index finger as fast as they could. The aim was to see how much the tapping interfered with performance on the Continuous Performance Tests, and so performance on these dual tasks was compared to performance on the same tasks in experiment 1. In addition, a tapping task was administered as a single test. Subjects were asked to tap as fast as they could for 5 minutes in three separate blocks, with a rest between each block. Three measures were taken, and their average was used to provide a more reliable index of speed of tapping.
Results
Four types of data were analyzed: 1) reaction times, 2) omission errors, 3) commission errors, and 4) tapping data. All four types of data were submitted to ANOVA, with subject group (patients and comparison subjects) as the between-group factor. The within-group factors were task type (dual task versus single task) and the target probability (17% versus 50%). As before, data on omission and commission errors were log transformed.
Reaction times
Not surprisingly, there was a significant main effect of group (F=7.15, df=1, 48, p<0.01) due to patients’ slower response latencies. Further, there was a significant effect of task type (F=11.03, df=1, 48, p<0.05), in which performance on the single task was faster than when subjects engaged in the additional tapping task (i.e., dual task). Finally, there was a significant effect of target probability (F=6.82, df=1, 48, p<0.01) in which performance on the 50% target probability task was substantially more rapid. In the dual task, comparison subjects’ mean response latencies were 335 msec (SD=70) and 323 msec (SD=52) on the 17% and 50% target probability tasks, respectively, and patients’ mean response latencies were 398 msec (SD=107) and 379 msec (SD=107), respectively. There were no significant interactions.
Omission errors
As expected, there was a significant main effect of group (F=17.58, df=1, 48, p<0.001) due to patients’ making more omissions overall. There was also a main effect of task type in which more omission errors were made on the Continuous Performance Test when subjects had to engage in a tapping task simultaneously (i.e., dual task) (F=8.84, df=1, 48, p<0.005). Finally, subjects made more omission errors when the task had a 50% target probability (F=23.07, df=1, 48, p<0.001). In the dual task, comparison subjects’ mean number of omissions (not log transformed) was 0.27 (SD=0.40) and 1.92 (SD=2.59) on the 17% and 50% target probability tasks, respectively, and patients’ mean number of omissions was 2.08 (SD=1.85) and 5.83 (SD=6.12), respectively. There were no significant interactions.
Commission errors
As expected, there was a significant main effect of group (F=5.67, df=1, 48, p<0.05) due to patients’ making more commission errors overall. Further, there was a significant effect of target probability (F=26.77, df=1, 48, p<0.001), in which more commission errors were made on the 50% probability task. There was no significant main effect of task type (F=0.31, df=1, 48, p>0.50). In the dual task, comparison subjects’ mean number of commissions (not log transformed) was 0.35 (SD=0.65) and 1.09 (SD=1.04) on the 17% and 50% target probability tasks, respectively, and patients’ mean number of commissions was 1.67 (SD=2.11) and 3.94 (SD=5.76), respectively. There were no significant interactions.
Tapping data
The data on tapping alone were subjected to an ANOVA, with group (patients and comparison subjects) as the between-group factor and block number (block 1, 2, or 3) as the within-group factor. Not surprisingly, comparison subjects were able to tap more times in five minutes than the patients (mean=1392 times [SD=131] versus 1122 times [SD=191]; F=35.23, df=1, 48, p<0.001).
The tapping data from the dual task were also subjected to an ANOVA, with group (patients and comparison subjects) as the between-group factor and concurrent task type as the within-group factor (17% and 50%). Not surprisingly, comparison subjects were able to tap more than the patients (mean of 886 times [SD=98] versus 654 times [SD=153]; F=56.46, df=1, 48, p<0.001). There was no significant difference between the task type that was concurrent with the tapping task (F=1.21, df=1, 48, p>0.10), nor was there a significant interaction of group and task type (F=0.69, df=1, 48, p>0.10).
Finally, as expected, an ANOVA that included all tapping data (in which blocks 1, 2, and 3 of the tapping only condition were averaged, as were the data on tapping from the dual task) revealed a significant group effect (F=51.45, df=1, 48, p<0.001) and a main effect of task (tapping alone versus tapping in the dual condition) (F=829.24, df=1, 48, p<0.001). However, there was no significant interaction of group and task (F=1.21, df=1, 48, p>0.1). Although tapping in the dual task was slower than in the task involving tapping alone, the difference was not disproportionate for patients with schizophrenia (in the dual task, mean=886 taps [SD=88] for comparison subjects versus 654 taps [SD=153] for patients; in the tapping alone task, mean=1392 taps [SD=131] for comparison subjects versus 1122 taps [SD=191] for patients).
Discussion
In this study we tested four possible mechanistic explanations of impaired performance on the Continuous Performance Test in patients with schizophrenia, yet we found no support for any of these hypothesized factors. First, we manipulated the amount of stimulus-response mapping. This procedure resulted in superior reaction times on the “ready-press” task compared with the “one-nine” task (i.e., the manipulation worked). However, it did not produce a disproportionate deficit in performance in patients with schizophrenia. Second, we increased the target probability to bias subjects to make responses. We expected that patients with schizophrenia would have disproportionate problems in inhibiting responses. Again, the manipulation worked in the sense that a difference in performance between the two tasks was found. However, despite previous research indicating that patients with schizophrenia have such inhibition problems, we found no evidence of this in our study. Third, we manipulated the length of time that subjects had to hold a previous stimulus in working memory. Again, although performance on the two tasks (SOA=1000 msec and SOA=3000 msec) was different, the patients with schizophrenia in our study did not show the expected (and previously found) decrement in performance as the SOA increased. Finally, we manipulated the amount of available motor planning resources available by adding a dual task component to the test (having subjects tap concurrently). Although, this manipulation worsened performance marginally, again there was not a group difference. To summarize, none of the four manipulations we employed produced the predicted differences in the performance of patients with schizophrenia on the Continuous Performance Test. However, it is important to keep in mind that patients’ performance on the test tasks (accuracy or reaction time) was always worse than comparison subjects’ performance (see below).
We suggest three possible reasons why we did not find differential changes in performance on the Continuous Performance Tests in the patients with schizophrenia. First, the findings may simply have resulted from a cohort effect, i.e., the patients in our study were not representative of the population of patients with schizophrenia (see, for example, reference 6, unpublished 1998 manuscript of Elvevåg). However, we argue that this probably was not the case, because the patients in our study had performed badly on other frequently used Continuous Performance Test tasks, such as the 1–9 (digit) task in the Gordon Systems (GDS-Model II) (16). Furthermore, our sample size was adequate (patient N=20) and the diagnosis was reliably established by SCID (DSM-IV). Most important, the patients in our study by no means performed normally; their performance was consistently and significantly less accurate and slower than that of comparison subjects. Thus, we contend that previous findings may not be as reliably replicable as we expected.
Second, is it possible that we failed to operationalize the variables appropriately, even though each variable produced a significant main effect in terms of reaction time (“ready-press” only) and omission or commission errors. Manipulating stimulus-response mapping affected performance on the Continuous Performance Test but did not affect performance differently in the two groups. Regarding SOA, it is possible that if we had made the interval longer, to make the task disproportionately more difficult for patients, a difference between the groups would have emerged. However, previous research has shown that the performance of patients with schizophrenia suffers disproportionately even at a delay period of 2000 msec (e.g., unpublished 1998 manuscript of Elvevåg). Alternatively, it may be that the target probability was not high enough. In other variants of the Continuous Performance Test it is possible to set the cue-target ratio at 80%. A study by Servan-Schreiber et al. (6) found that unmedicated patients with schizophrenia have a disproportionate problem overriding their overlearned response tendencies at long delay periods, compared to normal subjects and patients with schizophrenia who have received medication. In the Continuous Performance Test task used in this study, it was not possible to increase the target probability much higher than 50% without producing an obvious and predictable pattern of stimuli. It is possible that this procedure did not build up a response tendency as strong as the one that would have resulted from a task that required subjects to respond to every stimulus rather than just to the stimuli that called for “yes” responses. What is more probable is that building up response bias is not a particularly reliable way to elicit errors in patients with schizophrenia. Even Servan-Schreiber et al. (6), who proposed this parameter, failed to find that patients who were receiving medication were especially susceptible to errors on overlearned pairings at long SOAs (see also unpublished 1998 manuscript of Elvevåg for similar findings). Moreover, it is very important to note that Servan-Schreiber et al. employed psychiatric inpatients (with depression) with unreported medicated status as comparison subjects. This being said, their general finding that all subjects performed better in the short SOA condition was not replicated in our study. However, we do not disagree with the conclusion of Servan-Schreiber et al. that delayed response deficits are an important component of cognitive impairments in schizophrenia. Certainly these deficits have been reliably observed in a variety of paradigms (e.g., references 17–22). Rather, we posit that in the “classic” Continuous Performance Test (relative to low target probability and short SOA) different factors lie behind poor performance.
Third and finally, the dual task component did not interfere with performance on the Continuous Performance Test as much as we expected and, most important, did not do so differentially. Perhaps this result occurred because much of the processing of Continuous Performance Test tasks takes place in cortical association areas, not in the motor system. If so, it is possible that we needed to employ a more complex concurrent task, such as arithmetic problem solving.
We believe that our data suggest that patients’ impairments on the Continuous Performance Test are due to encoding problems. If this is so, a shorter, rather than a longer, SOA might have a differential impact on the performance of patients with schizophrenia. Certainly this explanation is consistent with the high sensitivity shown on versions of the Continuous Performance Test in which the stimuli are degraded (e.g., reference 23) or in which the stimulus duration is very brief (e.g., reference 24). The significant interaction effect of group, target probability (low), and SOA (short) on omission errors is consistent with the idea of an encoding deficit in patients with schizophrenia. For example, if patients were slow in constructing a representation, a short SOA would more likely lead to interference, as a second stimulus would exact a cost on ongoing processes. Such a situation would result in patients’ making relatively more omission errors, but patients thus might have a more conservative response bias in environments in which much information is not salient (e.g., the low target probability condition). It is noteworthy that studies using EEG (25) and pharmacological interventions in monkeys (26) have shown that “imprecision” in encoding can account for much of the variance in simple attention and working memory tasks. It is interesting to note that the anterior cingulate, thought to play a key role in attention, also appears to become preferentially active under difficult encoding conditions (27).
The study reported here produced unexpected negative results. Although group differences were large because patients made more errors and were slower, experimental parameters designed to manipulate response readiness, stimulus-response mapping, and working memory demands had little differential impact on patients. Thus, simple notions about Continuous Performance Test failure do not appear to account for the performance of patients with schizophrenia, at least in this study group. However, the results may still be heuristic because they indicate that in certain situations or conditions (of low target probability and short SOA), patients appear to have difficulty encoding (i.e., constructing a representation) and making rapid subsequent decisions of whether to respond.
The Continuous Performance Test provides clinicians and researchers with a valuable tool to index one of the key cognitive deficits characteristic of schizophrenia. The usefulness of this task is not in question. What our study does question is the explanatory power of certain contemporary mechanistic accounts of performance on the Continuous Performance Test, most notably our own. Perhaps Continuous Performance Test omissions represent a frank failure of patients’ ability to encode stimuli, due either to network shutdown or increases in background noise.
 |
 |
 |
Received Feb. 23, 1999; revision received Dec. 7, 1999; accepted Dec. 9, 1999. From the Clinical Brain Disorders Branch, NIMH. Reprints are not available. Address correspondence to Dr. Elvevåg, Clinical Brain Disorders Branch, NIMH, 10 Center Drive, Rm. 4C215, MSC 1373, Bethesda, MD 20892; [email protected] (e-mail).
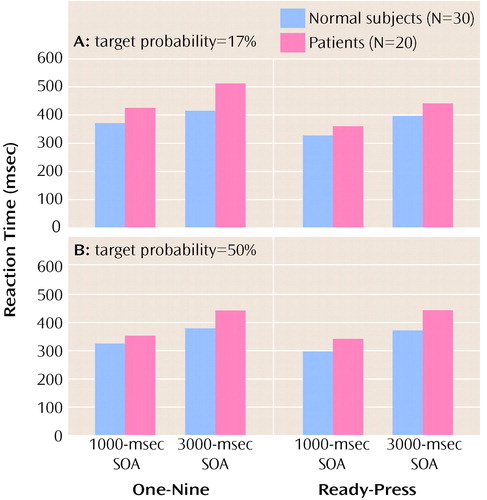
Figure 1. Mean Reaction Times for Normal Comparison Subjects and Patients With Schizophrenia on Two Types of Cognitive Tasks (One-Nine Task and Ready-Press Task) at Two Stimulus Onset Asynchronies (SOAs) and With Two Target Probabilities
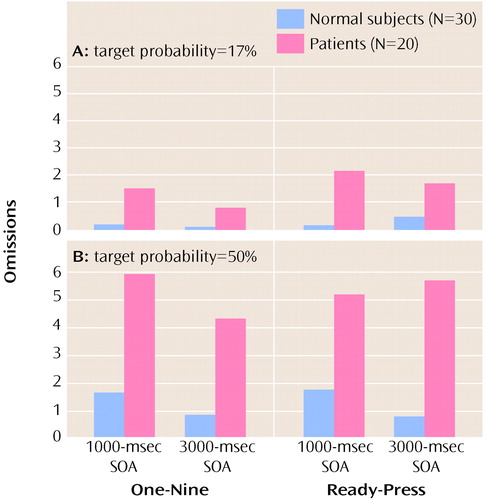
Figure 2. Mean Number of Omissions Made by Normal Comparison Subjects and Patients With Schizophrenia on Two Types of Cognitive Tasks (One-Nine Task and Ready-Press Task) at Two Stimulus Onset Asynchronies (SOAs) and With Two Target Probabilities
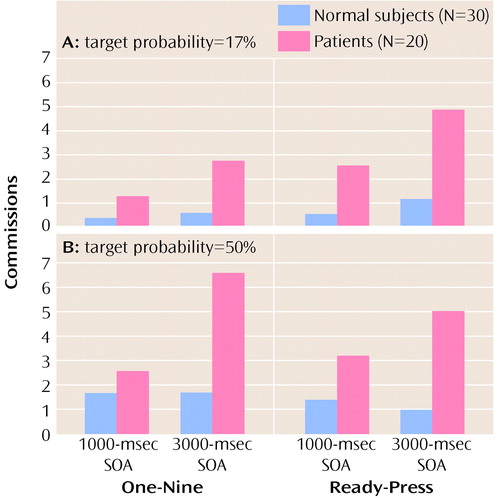
Figure 3. Mean Number of Commissions Made by Normal Comparison Subjects and Patients With Schizophrenia on Two Types of Cognitive Tasks (One-Nine Task and Ready-Press Task) at Two Stimulus Onset Asynchronies (SOAs) and With Two Target Probabilities
1. Shakow D: Segmental set: a theory of the formal psychological deficit in schizophrenia. Arch Gen Psychiatry 1962; 6:17–33Crossref, Google Scholar
2. Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Beck LH: A continuous performance test of brain damage. J Consult Psychol 1956; 20:343–350Crossref, Medline, Google Scholar
3. Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L: The Continuous Performance Test, Identical Pairs Version (CPT-IP), I: new findings about sustained attention in normal families. Psychiatry Res 1988; 26:223–238Crossref, Medline, Google Scholar
4. Nuechterlein KH, Dawson ME: Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 1984; 10:160– 203Crossref, Medline, Google Scholar
5. Cohen JD, Servan-Schreiber D: Context, cortex and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev 1992; 99:45–77Crossref, Medline, Google Scholar
6. Servan-Schreiber D, Cohen JD, Steingard S: Schizophrenic deficits in the processing of context: a test of a theoretical model. Arch Gen Psychiatry 1996; 53:1105–1112Google Scholar
7. Frith CD, Done DJ: Routes to action in reaction time tasks. Psychol Res 1986; 48:169–177Crossref, Medline, Google Scholar
8. Elvevåg B, Duncan J, McKenna PJ: The use of cognitive context in schizophrenia: an investigation. Psychol Med (in press)Google Scholar
9. Brown VJ, Robbins TW: Simple and choice reaction time performance following unilateral striatal dopamine depletion in the rat: impaired motor readiness but preserved response preparation. Brain 1991; 114:513–525Crossref, Medline, Google Scholar
10. Jastak S, Wilkinson GS: The Wide Range Achievement Test, Revised. Wilmington, Del, Jastak Associates, 1984Google Scholar
11. Wechsler D: Wechsler Adult Intelligence Scale—Revised. San Antonio, Tex, Psychological Corp (Harcourt), 1981Google Scholar
12. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
13. Gordon M, Mettleman BB: Technical Guide to the Gordon Diagnostic System (GDS). DeWitt, NY, Gordon Systems, 1987Google Scholar
14. Cox DR, Snell EJ: Analysis of Binary Data, 2nd ed. London, Chapman & Hall, 1989Google Scholar
15. Bertelson P: The time course of preparation. Q J Exp Psychol 1967; 19:272–279Crossref, Medline, Google Scholar
16. Weickert TW, Goldberg TE, Gold FM, Bigelow LB, Egan MF, Weinberger DR: Differential patterns of cognitive impairment in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry (in press)Google Scholar
17. Park S, Holzman PS: Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 1992; 49:975–982Crossref, Medline, Google Scholar
18. Fleming K, Goldberg TE, Gold JM, Weinberger DR: Verbal working memory dysfunction in schizophrenia: use of a Brown-Peterson paradigm. Psychiatry Res 1995; 56:155–161Crossref, Medline, Google Scholar
19. Fleming K, Goldberg TE, Binks S, Randolph C, Gold JM, Weinberger DR: Visuospatial working memory in patients with schizophrenia. Biol Psychiatry 1997; 41:43–49Crossref, Medline, Google Scholar
20. Harvey PD, Keefe RS, Moskowitz J, Putnam KM, Mohs RC, Davis KL: Attentional markers of vulnerability to schizophrenia: performance of medicated and unmedicated patients and normals. Psychiatry Res 1990; 33:179–188Crossref, Medline, Google Scholar
21. Goldberg TE, Patterson KJ, Taqqu Y, Wilder K: Capacity limitations in short-term memory in schizophrenia: tests of competing hypotheses. Psychol Med 1998; 28:665–673Crossref, Medline, Google Scholar
22. Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR: Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry 1997; 54:159–165Crossref, Medline, Google Scholar
23. Nuechterlein KH, Parasuraman R, Jiang Q: Visual sustained attention: image degradation produces rapid sensitivity decrement over time. Science 1983; 220:327–329Crossref, Medline, Google Scholar
24. Cornblatt BA, Keilp JG: Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull 1994; 20:31–46Crossref, Medline, Google Scholar
25. Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N: Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J Abnorm Psychol 1997; 106:315–324Crossref, Medline, Google Scholar
26. Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC: Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci USA 1996; 93:11962– 11967Google Scholar
27. Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC: A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 1997; 5:49–62Crossref, Medline, Google Scholar



