High Levels of Dopamine Activity in the Basal Ganglia of Cigarette Smokers
Abstract
OBJECTIVE: The authors’ goal was to study presynaptic dopamine activity in smoking and nonsmoking human subjects in vivo. METHOD: [18F]Fluorodopa ([18F]DOPA) uptake Ki values in the basal ganglia of nine smoking and 10 nonsmoking healthy men were measured with positron emission tomography. RESULTS: Significantly higher [18F]DOPA uptake was observed in both the putamen (average 17.3% higher) and the caudate (average 30.4% higher) of smokers than in those of nonsmokers. CONCLUSIONS: Smoking is related to greater dopamine activity in the human basal ganglia. Nicotine-induced dopamine activity may be a relevant mechanism in dependence on cigarette smoking.
Cigarette smoking is a great health problem in the general population (1). It is extremely prevalent among patients with schizophrenia (2). Smoking is associated with a lower risk of Parkinson’s disease and neuroleptic-induced parkinsonism (3). Both the addictive effects and the controversial associations with schizophrenia and Parkinson’s disease are related to dopamine release in the central nervous system (CNS) (1–3).
In animal studies, nicotine, the neuroactive compound of tobacco, releases dopamine and stimulates energy metabolism in the basal ganglia, and especially in the ventral tegmental area and nucleus accumbens (4), which have been suggested to be central in reinforcing the effect of addictive compounds (5). In a human study, intravenously administered nicotine induced a dose-dependent increase in neuronal activity in a distributed system of brain regions, including the nucleus accumbens, amygdala, cingulate, and frontal lobes (6).
There is, however, no direct evidence of smoking-induced or nicotine-induced increases in dopamine transmission in the human CNS. On the contrary, Geracioti et al. (7) found that smokers had markedly lower dopamine metabolite homovanillic acid (HVA) levels than nonsmokers in the CSF but not in the plasma. Our aim was to study whether presynaptic dopamine transmission, measured by [18F]fluorodopa ([18F]DOPA) uptake with positron emission tomography (PET) techniques, is increased in the basal ganglia of healthy smoking men.
METHOD
The subjects were white Caucasian volunteers, 35 to 40 years old; all were healthy men working in a local shipyard. The smokers (N=9) had smoked cigarettes for at least the 15 previous years (mean=19.8 years, range=15–25) and smoked currently at least 15 cigarettes/day (mean=19.8 cigarettes/day, range=16–25). The age-matched comparison group (N=10) had never smoked regularly and had not smoked at all during the previous 5 years. There was no significant difference between smokers and nonsmokers in age (mean=37.1 years, SD=3.4, versus mean=36.8 years, SD=4.1) and body mass index (mean=24.8, SD=1.9, versus mean=24.0, SD=2.6).
All subjects underwent a physical and neuropsychological examination and psychiatric interview, including a standardized psychiatric interview (the Schedules for Clinical Assessment in Neuropsychiatry [8]) and laboratory tests. Only subjects who had no CNS disease or trauma or DSM-IV psychiatric diagnosis and who were found to be healthy on physical examination were selected for the study.
The study protocol was approved by the Ethical Committee of Turku University/Turku University Central Hospital, Finland. After complete description of the study to the subjects, written informed consent was obtained.
Dopamine activity was measured by using [18F]DOPA PET techniques. All subjects were allowed to have a light breakfast at about 8:00 a.m.; no food was allowed 3–4 hours before the PET scan. The smokers were allowed to smoke as usual 2 to 3 hours before the PET scan. [18F]DOPA preparation and high performance liquid chromatography analysis of unchanged [18F]DOPA and labeled metabolites in arterial plasma were performed as described elsewhere (9). The PET experiments were performed by using a whole-body PET scanner (ECAT 931/08-12) and analyzed as described elsewhere (10). The statistical analysis was done with a two-way repeated measures analysis of variance (ANOVA) and Student’s t test.
RESULTS
The Ki values for dopamine derived from a graphical analysis are summarized in FIGURE 1.. The average value was 17.3% higher for smokers (mean=0.013, SD=0.002) than nonsmokers (mean=0.011, SD=0.001) in the putamen (t=2.17, df=17, p=0.04) and 30.4% higher for smokers (mean=0.010, SD=0.003) than nonsmokers (mean=0.008, SD=0.002) in the caudate (t=2.31, df=17, p=0.03). Differences between smokers and nonsmokers were also statistically significant in the left putamen (17.5%) and right caudate (38.2%) (FIGURE 1.). Two-way ANOVA revealed no significant group-by-hemisphere interactions in the caudate (F=3.31, df=1, 17, p=0.09) or in the putamen (F=0.03, df=1, 17, p=0.87). The main effect of group was significant both in the caudate (F=5.36, df=1, 17, p=0.03) and in the putamen (F=4.65, df=1, 17, p=0.05).
DISCUSSION
Striatal dopamine activity, measured by [18F]-DOPA uptake, proved to be significantly higher in smokers than nonsmokers. To our knowledge, this is the first time it has been shown in vivo that cigarette smoking is related to increased dopaminergic activity in human basal ganglia.
As a central component of cigarette smoke, nicotine increases the release of dopamine in the smoker’s striatum and consequently can also stimulate the presynaptic dopamine synthesis in the striatum.
It has been reported that striatal monoamine oxidase A and B (MAO-A and MAO-B) levels in smokers are reduced (11, 12). Given that dopamine is metabolized in the brain by both MAO-A and MAO-B (13), our results on high [18F]DOPA Ki values in smokers are in good agreement with the MAO inhibition finding. MAO is a predominantly intracellular enzyme; therefore, more dopamine may be directed to the synthesis pathway rather than to transformation to 3,4-dihydroxyphenylacetic acid. The low CSF HVA in smokers (7) is also well explained by reduced MAO levels inhibiting both metabolic pathways (MAO-catechol O-methyltransferase [COMT] and COMT-MAO) from dopamine to HVA. The low CSF HVA does not appear to index presynaptic dopamine function. Our measurements suggest that smoking and the development of nicotine dependence are associated with increased dopamine activity in the basal ganglia.
It is also possible that smokers form a group of people with a special neurophysiological sensitivity to presynaptic dopaminergic activation caused by nicotine. The finding that type 1 abstinent alcoholics also had high [18F]DOPA uptake in the basal ganglia (14) suggests that nicotine and alcohol dependence may have a similar dopamine-related mechanism.
Received March 3, 1999; revision received Sept. 9, 1999; accepted Sept. 15, 1999. From the Department of Psychiatry and the Department of Diagnostic Radiology, Turku University Central Hospital; the Department of Pharmacology and Clinical Pharmacology, University of Turku; and the Turku PET Center, Accelerator Laboratory. Address reprint requests to Dr. Salokangas, Department of Psychiatry, Turku University Central Hospital, FIN-20520 Turku, Finland; [email protected] (e-mail). Supported by the University of Turku and the Turku University Central Hospital. The authors thank the Kvaerner Masa-Yards, its health station staff, and the shipyard workers who participated in the study.
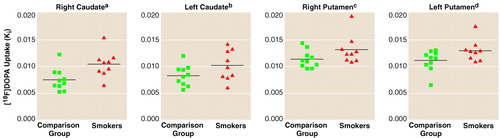
FIGURE 1. Scatterplots of Striatal [18F]Fluorodopa Ki Values of Nine Smokers and 10 Nonsmokers
at=2.78, df=17, p=0.01.
bt=1.72, df=17, p=0.10.
ct=1.79, df=17, p=0.09.
dt=2.12, df=17, p=0.05.
1. Iversen LL: Smoking harmful to the brain. Nature 1996; 382:206–207Crossref, Medline, Google Scholar
2. Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA: Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 1986; 143:993–997Link, Google Scholar
3. Decina P, Caracci G, Sandik R, Berman W, Mukherjee S, Scapicchio P: Cigarette smoking and neuroleptic-induced parkinsonism. Biol Psychiatry 1990; 28:502–508Crossref, Medline, Google Scholar
4. Pontieri FE, Tanda G, Orzi F, DiChiara G: Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996; 382:255–257Crossref, Medline, Google Scholar
5. Koob GF: Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 1992; 13:177–184Crossref, Medline, Google Scholar
6. Stein EA, Pankiewicz J, Harsch HH, Cho J-K, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS: Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 1998; 155:1009–1015Google Scholar
7. Geracioti TD Jr, West SA, Baker DG, Hill KK, Ekhato NN, Wortman MD, Keck PE Jr, Norman AB: Low CSF concentration of dopamine metabolite in tobacco smokers. Am J Psychiatry 1999; 156:130–132Link, Google Scholar
8. Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, Jablenski A, Regier D, Sartorius N: SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 1990; 47:589–593Crossref, Medline, Google Scholar
9. Bergman J, Haaparanta M, Lehikoinen P, Solin O: Electrophilic synthesis of 6-[18F]-fluoro-l-dopa, starting from aqueous [18F]-fluoride. J Labelled Compounds and Radiopharmaceuticals 1994; 35:476–477Google Scholar
10. Hietala J, Syvã«¡hti E, Vilkman H, Vuorio K, R㪫�nen V, Bergman J, Haaparanta M, Solin O, Kuoppam㪩 M, Eronen E, Ruotsalainen U, Salokangas RKR: Domains of psychopathology and presynaptic dopamine function in vivo in neuroleptic-naive schizophrenia. Schizophr Res 1999; 35:41–50Crossref, Medline, Google Scholar
11. Fowler JS, Volkow ND, Wang G-J, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer D, Zezulkova I, Wolf AP: Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci USA 1996; 93:14065–14069Google Scholar
12. Fowler JS, Volkow ND, Wang G-J, Pappas N, Logan J, MacGregor RR, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R: Inhibition of monoamine oxidase B in the brains of smokers. Nature 1996; 379:733–736Crossref, Medline, Google Scholar
13. Glover V, Elsworth JD, Sandler M: Dopamine oxidation and its inhibition by (-)-depryls in man. J Neural Transm Suppl 1980; 16:163–172Medline, Google Scholar
14. Tiihonen J, Vilkman H, Rã´¤nen P, Ryynã¥n O-P, Hakko H, Bergman J, H㮤l㨮en T, Laakso A, Haaparanta-Solin M, Solin O, Kuoppam㪩 M, Syvã«¡hti E, Hietala J: Striatal presynaptic dopamine function in type 1 alcoholics measured with positron emission tomography. Mol Psychiatry 1998; 4:156–161Crossref, Google Scholar



