Auditory Startle Response in Trauma Survivors With Posttraumatic Stress Disorder: A Prospective Study
Abstract
OBJECTIVE: Previous studies have shown elevated autonomic responses to startling tones in trauma survivors with chronic posttraumatic stress disorder (PTSD). The origin of these abnormal responses is obscure. The present study attempted to clarify this issue by prospectively evaluating responses to sudden, loud tones in individuals who arrived at a hospital emergency room after psychologically traumatic events. METHOD: By using a previously established protocol, autonomic and muscular responses to the tones were evaluated at 1 week, 1 month, and 4 months after the traumatic event. Structured diagnostic interviews performed at 4 months classified subjects into groups with (N=36) and without (N=182) PTSD, which were further subdivided according to the presence or absence of major depressive disorder as follows: neither PTSD nor depression (N=166), depression alone (N=16), PTSD alone (N=21), and both PTSD and depression (N=15). RESULTS: The groups showed comparable physiological responses to the tones at 1 week posttrauma. However, at 1 and 4 months posttrauma, the subjects with PTSD showed a greater heart rate response and required more stimulus trials to reach the criteria of skin conductance and orbicularis oculi electromyogram nonresponse. These findings were not significantly influenced by comorbid depression and were not explained by the severity of the traumatic event or by the intensity of the initial symptoms. CONCLUSIONS: Differences in physiological response to startling tones develop along with PTSD in the months that follow a traumatic event. This pattern supports the theories that associate PTSD with progressive neuronal sensitization.
Despite the growing evidence of biological dysfunction in chronic posttraumatic stress disorder (PTSD) (1, 2), the pathogenesis of the disorder remains poorly understood. A preponderance of individuals report the appearance of some symptoms of PTSD shortly after the occurrence of traumatic events (3). With time, these symptoms abate in most trauma survivors, but some are left with full-blown PTSD (4). Among the early symptoms, self-reports of exaggerated startle response are sensitive predictors of the PTSD outcome (5). Exaggerated startle response is also a DSM-IV diagnostic criterion for the disorder.
The neurobiology of the acoustic startle reflex has been studied extensively in animals (6, 7). In humans, this reflex is typically measured as the magnitude of the muscular eye blink response to sudden, loud tones or noises. However, cardiac acceleration and increased electrodermal conductivity of longer latency and duration also regularly follow startle-generating stimuli (8). The muscular and electrodermal responses typically decline (i.e., habituate) upon repeated presentations of the same stimulus (9). Elevated heart rate response to startling stimuli represents a highly consistent finding in PTSD; larger skin conductance and orbicularis oculi (eye blink) electromyographic (EMG) responses, and slower skin conductance and EMG response habituation, have also been found (10–15).
In healthy humans, about 40% of the variance in skin conductance habituation is inherited (16). Moreover, slower skin conductance habituation, as measured by the number of stimulus trials required to reach a nonresponse criterion, correlates with heightened autonomic conditionability (17). One might hypothesize that the larger, more slowly habituating physiologic responses to startling tones found in trauma survivors with PTSD reflect inherited vulnerability (18). In this case, they should be present before the traumatic event in individuals who go on to develop this disorder.
Alternatively, one might hypothesize that the abnormal physiological startle response seen in trauma survivors may develop, along with PTSD, after traumatic exposure as a consequence of a progressive sensitization of the central nervous system (CNS) (2, 19–21). In this case, the abnormal startle response should not be present before PTSD develops. Both hypotheses presume that abnormal startle response is specific to PTSD and is not present in the subjects who develop other disorders after psychological trauma.
This study prospectively evaluated physiological response to startling stimuli in 218 individuals who had just experienced an acute, emotionally traumatic event and were classified according to whether or not they subsequently developed PTSD and/or major depressive disorder.
METHOD
Subjects
The data presented here were part of a large-scale prospective investigation of the psychiatric effects of acute psychological trauma (22–24). As previously described, patients arriving at the Hadassah University Hospital emergency room in Jerusalem after a traumatic event were recruited into the study over 3 years. The patients were examined by a research psychologist (T.P., D.B., or S.F.) and considered for the study if they were between ages 16 and 65 years and had experienced an event meeting DSM-III-R criterion A for PTSD.
Subjects were not invited to participate if they reported a history of having regularly used drugs or alcohol, suffered from a past or present psychotic condition, had previously been diagnosed as suffering from PTSD, experienced a head injury from the current trauma, lost consciousness, developed a severe medical or surgical condition, or were exposed to ongoing victimization (e.g., domestic violence) that could interfere with recovery from the index trauma.
The subjects received information about the study and gave written informed consent. They were subsequently evaluated at 1 week, 1 month, and 4 months after the traumatic event. These evaluations included structured clinical interviews, psychometric tests, and laboratory assessments of physiological response to sudden, loud tones.
Of 430 candidates seen in the emergency room, 275 agreed to participate, and 236 (86%) completed all three evaluation sessions. Eighteen subjects were excluded because of incomplete physiological data due to technical problems, leaving 218 subjects with valid data. The 218 subjects who completed the study and the 57 who did not had similar mean ages and gender distributions. The noncompleters tended to report lower symptom levels at 1 week (raw data available from Dr. Shalev).
The traumatic events experienced by the subjects in the current study included motor vehicle accidents (85%, N=185), terrorist attacks (7%, N=15), work accidents (4%, N=8), witnessing violence (1%, N=3), and miscellaneous traumatic events (3%, N=7). The survivors of motor vehicle accidents did not significantly differ from all other subjects on their trauma severity scores (explained later): mean=4.9, SD=1.6, versus mean=5.0, SD=1.5, respectively (t<1, df=216, n.s.).
The Clinician-Administered PTSD Scale (25) was used to assess PTSD symptoms, and the Structured Clinical Interview for DSM-III-R (26) was used to assess the symptoms of major depressive disorder and other axis I mental disorders at 4 months posttrauma. On the basis of these interviews, the subjects were classified into groups with (N=36) and without (N=182) PTSD, which were further subdivided according to the presence or absence of major depressive disorder as follows: neither PTSD nor depression (N=166, 87 men and 79 women; 140 in motor vehicle accidents and 26 with other traumatic events), depression alone (N=16, 13 men and three women; 15 in motor vehicle accidents and one with another traumatic event), PTSD alone (N=21, 11 men and 10 women; 18 in motor vehicle accidents and three with other traumatic events), and both PTSD and depression (N=15, five men and 10 women; 13 in motor vehicle accidents and two with other traumatic events). There were no significant associations between the presence or absence of PTSD or depression and gender or event type (motor vehicle accident versus other type of traumatic event); for all Mantel-Haenszel chi-square tests, p>0.10.
Psychometrics
Psychometric questionnaires included the Peritraumatic Dissociative Experiences Questionnaire (27), the Impact of Event Scale (28), the State-Trait Anxiety Inventory (29), the Mississippi Scale for Combat-Related PTSD, civilian version (30), and the Beck Depression Inventory (31). Detailed analyses of the psychometric properties of these instruments have been published previously (22–24).
Twelve mental health professionals who were blind to the subjects’ PTSD status reviewed an audiotaped script describing each traumatic event (32, 33) and independently rated its severity on a scale of 1–10 (1=not severe at all, 10=extreme severity). Raters took into consideration such generic dimensions of trauma as a direct threat to self or others, exposure to injury or death of close relatives, and exposure to human disfigurement or death. The ratings of the 12 observers were averaged for each subject before the statistical analyses.
Physiological Responses
Left orbicularis oculi EMG, nondominant palmar skin conductance, and heart rate response to a series of 15 95-dB (SPL), 1000-Hz, 500-msec pure tones with 0-msec rise and fall times presented over headphones were assessed according to a previously described technique (11–15) and in accordance with published recommendations (34). Intertrial intervals were randomly selected and ranged from 30 to 55 seconds. Sampling of the physiological measures was initiated 4 sec before each tone presentation at the rate of 50 Hz and continued until 8.5 seconds after the onset of each tone.
Procedure
After recruitment from the emergency room, the subjects attended follow-up evaluations at 1 week (mean=7.7 days, SD=3.8), 1 month (mean=33.0 days, SD=6.1), and 4 months (mean=117.7 days, SD=6.1) after the traumatic event. At the 1-week follow-up session, each subject was administered the Impact of Event Scale, the State-Trait Anxiety Inventory state scale, the Beck Depression Inventory, and the Peritraumatic Dissociative Experiences Questionnaire. At the 1- and 4-month sessions, the Impact of Event Scale, the State-Trait Anxiety Inventory state scale, the Beck Depression Inventory, the Mississippi Scale for Combat-Related PTSD, and the Clinician-Administered PTSD Scale were administered. Physiological response to the tones was evaluated during each follow-up session and immediately after the psychodiagnostic/psychometric evaluation. Before exposure to the tones, each subject was instructed as follows: “You are going to hear a series of sounds. Please sit quietly and listen to the sounds as they come. Keep your eyes open throughout the entire procedure, which will not last more than 15 minutes.”
Data Analysis
An EMG response score for each trial was calculated by subtracting the average baseline EMG level for the 1 second immediately preceding the onset of the tone from the maximum EMG level within 40 to 200 msec of the tone onset. A subject was considered to have reached the EMG nonresponse criterion (i.e., become habituated) after two consecutive EMG responses of <0.1 µV. The possible range of this “trials to nonresponse” variable was 0 (nonresponse on each of the first two trials) to 14 (no two consecutive nonresponse trials). A skin conductance response score was calculated for each trial by subtracting the average baseline skin conductance level for the 1 second immediately preceding the tone onset from the maximum skin conductance level within 1 to 4 seconds after the tone onset. A subject was considered to have reached the skin conductance nonresponse criterion after two consecutive skin conductance responses of <0.05 µS.
A heart rate response score was calculated in the same manner as was the skin conductance response score. On the basis of previous results, no decrease in heart rate response over the trials was predicted (nor observed). Hence, the number of trials needed to reach the heart rate nonresponse criterion was not calculated.
To reduce the variance associated with unusually large responses, square root transformations were performed on the EMG, skin conductance, and heart rate response scores before the statistical analysis. The data were subjected to analyses of variance and covariance according to the general linear model. A p value of <0.05 conferred statistical significance. For main effects and interactions involving the session factor (1 week, 1 month, or 4 months), p values were subjected to a Greenhouse-Geisser correction.
RESULTS
Demographics, Psychodiagnostics, and Psychometrics
Table 1 presents demographic and psychometric data for the study groups over 4 months. There were no significant differences among the groups with regard to age or education. The severity of the traumatic events was rated higher in subjects with than without depression. Subjects with PTSD reported more peritraumatic dissociation. Self-reported symptoms on the Impact of Event Scale were higher and decreased less across sessions in subjects with PTSD, especially those with comorbid depression. State anxiety and depressive symptoms and scores on the Clinician-Administered PTSD Scale and the Mississippi Scale for Combat-Related PTSD were greater in subjects with than without PTSD and with than without depression. State anxiety and depressive symptoms decreased across sessions, but scores on the Mississippi Scale for Combat-Related PTSD did not. Scores on the Clinician-Administered PTSD Scale decreased across sessions (although not appreciably in subjects with both PTSD and depression).
Physiological Measures
Table 2 presents the heart rate, skin conductance, and EMG responses over the 15 tone trials and the number of trials needed to reach the nonresponse criteria for skin conductance and EMG for the four groups. Figure 1 presents these data for the subjects with and without PTSD, collapsed across depression. A significant PTSD-by-session interaction for heart rate response (F=3.9, df=2, 428, p=0.02) was due to an increase over sessions in the subjects with PTSD. A significant PTSD-by-session interaction for the number of trials required to reach the skin conductance nonresponse criterion (F=3.8, df=2, 428, p=0.02) was due to a smaller decrease over the sessions in the subjects with PTSD. A significant PTSD-by-session interaction for trials to reach the EMG nonresponse criterion (F=3.2, df=2, 428, p=0.04) was due to an increase over the sessions in the subjects with PTSD and a decrease in the subjects without PTSD. The PTSD-by-session interactions for skin conductance and EMG response showed the same patterns as the number of trials required to reach the skin conductance and EMG nonresponse criteria, respectively, but were not statistically significant. There were no significant PTSD or depression main effects or PTSD-by-depression, depression-by-session, or PTSD-by-depression-by-session interactions for any physiological variable.
Analyses of covariance were performed to control for potentially confounding variables in the previously noted PTSD-by-session interactions for heart rate response and the number of trials required to reach the skin conductance and EMG nonresponse criteria. For the heart rate response, employing trauma severity as a single covariate yielded these data: F=3.3, df=2, 370, p=0.04. Adding the Peritraumatic Dissociative Experiences Questionnaire score as a second covariate yielded these results: F=2.9, df=2, 346, p=0.06. Adding Impact of Event Scale, State-Trait Anxiety Inventory, and Beck Depression Inventory scores at 1 week as the third, fourth, and fifth covariates yielded these data: F=2.4, df=2, 332, p=0.09. For the number of trials needed to reach the skin conductance nonresponse criterion, employing trauma severity, Peritraumatic Dissociative Experiences Questionnaire score, and 1-week scores on the Impact of Event Scale, state anxiety subscale, and Beck Depression Inventory as five covariates yielded these results: F=5.7, df=2, 332, p=0.004. For the number of trials needed to reach the EMG nonresponse criterion, employing trauma severity as a single covariate yielded these data: F=4.9, df=2, 370, p=0.01. Adding the Peritraumatic Dissociative Experiences Questionnaire score as a second covariate yielded these results: F=2.9, df=2, 346, p=0.06. Adding Impact of Event Scale, state anxiety, and Beck Depression Inventory scores at 1 week as the third, fourth, and fifth covariates yielded these data: F=2.5, df=2, 332, p=0.09.
DISCUSSION
This study prospectively evaluated physiological response to a series of startling tones in trauma survivors at 1 week, 1 month, and 4 months after they arrived at a general hospital emergency room after a psychologically traumatic event. At 4 months, they were assessed for PTSD and depression. At 1 week posttrauma, the responses of the groups were comparable. However, at 1 and 4 months posttrauma, the heart rate response to startling tones of the subjects with PTSD had become larger than those of the subjects without PTSD. The subjects with PTSD also showed less decline over the follow-up sessions than the subjects without PTSD in the number of trials required to reach the skin conductance nonresponse criterion, and they showed an increase in the number of trials needed to reach the EMG nonresponse criterion.
The results of this study are consistent with the second hypothesis—i.e., that abnormally elevated response to startling tones develops along with PTSD. The findings were not significantly influenced by comorbid depression, and they were not fully explained by the rated severity of the traumatic event nor by the self-reported intensity of the early symptoms.
The pattern of group differences in physiological response in subjects with PTSD to the startling tones observed here at 1 and 4 months after the traumatic event resembles that previously found in subjects with chronic PTSD, including Vietnam veterans 20 years after combat (10, 12), Israeli civilians 10 years after exposure to a variety of traumatic events (11), Israeli prisoners of war 20 years after captivity (13), and adult women sexually abused as children (15). This similarity suggests that abnormal physiological response to startling stimuli may persist for as long as the disorder is expressed. However, these PTSD subjects’ mean skin conductance and EMG responses to the tones and the number of trials needed to reach the skin conductance and EMG nonresponse criteria were lower than those observed in the subjects with chronic PTSD in the previously cited studies. This discrepancy might be explained in several ways. First, across-session habituation may have affected the response of the subjects with PTSD here (35). However, the degree of across-session habituation was less in the subjects with than without PTSD. Second, physiological response to startling stimuli may continue to grow as PTSD becomes more chronic. Third, individuals who have PTSD at 4 months but then recover (3, 4) may not be included in the groups studied at a later stage of the disorder.
It seems implausible that the simple tone stimulus employed here could have been associated with the various traumatic events experienced by the subjects because of its generic nature. Hence, the observed responses cannot be explained as learned, conditioned responses. Rather, they seem to represent newly acquired responses (36) that either develop or fail to habituate between sessions in patients with PTSD during the months after a traumatic event. The absence of these response patterns in individuals who develop depression supports the occurrence of pathogenic processes unique to PTSD. The neuronal mechanisms that underlie these processes are unclear but may reflect a progressive neuronal sensitization leading to heightened responsivity (2, 19, 20) or an impaired capacity to correctly classify intense, yet redundant, auditory stimuli as harmless (21), which develops along with PTSD.
This study did not include data collected before the occurrence of the traumatic event. Consequently, the first hypothesis (i.e., that abnormal startle response precedes a trauma) has not been definitively ruled out. This can only be done by obtaining physiological measurements before the trauma. However, in light of the results obtained here, for this hypothesis to be viable, a greater heart rate response to the sudden, loud tones would have had to be present in the subjects at risk for PTSD before the traumatic event and the response would have had to disappear a week later and then reappear a month later.
Previous prospective studies of PTSD (22, 24) have indicated that the initial symptoms reported by many trauma survivors do not decay in those who go on to qualify for the diagnosis of PTSD, whereas they gradually disappear in trauma survivors without PTSD. Signs and symptoms of avoidance may increase over time in patients who show persistent hyperarousal, anxiety, and intrusive recollections of the traumatic event (37, 38). The present results appear to reflect a similar development, in that heightened response to sudden, loud tones became apparent in individuals who continued to express trauma-related symptoms months after the trauma. Epidemiological studies have shown that the likelihood of recovery from PTSD declines rapidly during the first year after exposure (4). This may be due to the induction of lasting changes in CNS routines of stimulus evaluation and responsivity, such as the ones captured by this study.
Our present knowledge is limited with regard to the relative contributions of the traumatic event and the immediate response to it versus the secondary external or internal reinforcing factors in the pathogenesis of PTSD. Secondary posttrauma stressors such as the interruption of interpersonal relationships or continuing physical pain may contribute to the results but were not evaluated in this study.
In a previous study (23), we found elevated heart rate levels at the time of arrival at an emergency room in uninjured trauma survivors who later developed PTSD. That finding suggested a link between immediate trauma response and subsequent PTSD. In contrast, the delayed appearance in the present study of a difference in heart rate response to startling tones in individuals who went on to develop PTSD points to a pathogenic process operative in the weeks and months after a psychological trauma. A better understanding of immediate and delayed biological response to traumatic events may lead to more effective secondary preventive interventions in PTSD.
Received Aug. 11, 1998; revisions received March 29 and July 12, 1999; accepted Aug. 12, 1999. From the Center for Traumatic Stress, Department of Psychiatry, Hadassah University Hospital; the Hebrew University Medical School, Jerusalem; the VA Research Service, Manchester, N.H.; and the Department of Psychiatry, Harvard Medical School, Boston. Address reprint requests to Dr. Shalev, Center for Traumatic Stress, Department of Psychiatry, Hadassah University Hospital, P.O. Box 12000, Jerusalem 91120, Israel; [email protected] (e-mail). Supported by NIMH grant MH-50379.
 |
 |
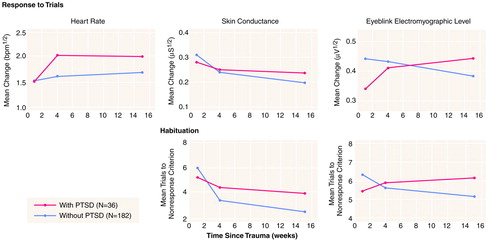
FIGURE 1. Physiological Responses to a Series of 15 Sudden, Loud Tones in Trauma Survivors With and Without PTSD
1. Pitman RK: Overview of biological themes in PTSD. Ann NY Acad Sci 1997; 821:1–9Crossref, Medline, Google Scholar
2. Yehuda R: Sensitization of the hypothalamic-pituitary-adrenal axis in posttraumatic stress disorder. Ann NY Acad Sci 1997; 821:57–75Crossref, Medline, Google Scholar
3. Rothbaum BO, Foa EB: Subtypes of posttraumatic stress disorder and duration of symptoms, in Posttraumatic Stress Disorder: DSM IV and Beyond. Edited by Davidson JRT, Foa EB. Washington, DC, American Psychiatric Press, 1993, pp 23–35Google Scholar
4. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048–1060Google Scholar
5. McFarlane AC: The phenomenology of posttraumatic stress disorders following a natural disaster. J Nerv Ment Dis 1988; 176:22–29Crossref, Medline, Google Scholar
6. Davis M: The mammalian startle response, in The Neural Mechanism of Startle Response. Edited by Eaton RC. New York, Plenum, 1984, pp 287–351Google Scholar
7. Davis M: Neural systems involved in fear-potentiated startle. Ann NY Acad Sci 1989; 563:165–183Crossref, Medline, Google Scholar
8. Turpin G: Effects of stimulus intensity on autonomic responding: the problem of differentiating orienting and defense reflexes. Psychophysiology 1986; 23:1–14Crossref, Medline, Google Scholar
9. Wilkins DE, Hallett M, Wess MM: Audiogenic startle reflex of man and its relationship to startle syndromes. Brain 1986; 109:561–573Crossref, Medline, Google Scholar
10. Paige SR, Reid GM, Allen MG, Newton JE: Psychophysiological correlates of posttraumatic stress disorder in Vietnam veterans. Biol Psychiatry 1990; 27:419–430Crossref, Medline, Google Scholar
11. Shalev AY, Orr SP, Peri T, Schreiber S, Pitman RK: Physiologic responses to loud tones in Israeli patients with posttraumatic stress disorder. Arch Gen Psychiatry 1992; 49:870–875Crossref, Medline, Google Scholar
12. Orr SP, Lasko NB, Shalev AY, Pitman RK: Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J Abnorm Psychol 1995; 104:75–82Crossref, Medline, Google Scholar
13. Orr SP, Solomon Z, Peri T, Pitman RK, Shalev AY: Physiologic responses to loud tones in Israeli veterans of the 1973 Yom Kippur War. Biol Psychiatry 1997; 41:319–326Crossref, Medline, Google Scholar
14. Shalev AY, Peri T, Orr SP, Bonne O, Pitman RK: Auditory startle responses in help-seeking trauma survivors. Psychiatry Res 1997; 69:1–7Crossref, Medline, Google Scholar
15. Metzger LJ, Orr SP, Berry NJ, Ahern CE, Lasko NB, Pitman RK: Physiologic reactivity to startling tones in women with posttraumatic stress disorder. J Abnorm Psychol 1999; 108:347–352Crossref, Medline, Google Scholar
16. Lykken DT, Iacono WG, Haroian K, McGue M, Bouchard TJ Jr: Habituation of the skin conductance response to strong stimuli: a twin study. Psychophysiology 1988; 25:4–15Crossref, Medline, Google Scholar
17. Öhman A, Bohlin G: Magnitude and habituation of the orienting reaction as predictors of discriminative electrodermal conditioning. J Experimental Res Personality 1973; 6:293–299Google Scholar
18. Shalev AY, Rogel-Fuchs Y: Psychophysiology of the posttraumatic stress disorder: from sulfur fumes to behavioral genetics. Psychosom Med 1993; 55:413–423Crossref, Medline, Google Scholar
19. Yehuda R, Antelman SM: Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry 1993; 33:479–486Crossref, Medline, Google Scholar
20. Post RM, Weiss SRB, Smith M: Sensitization and kindling: implications for the evolving neural substrates of post-traumatic stress disorder, in Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to Post-Traumatic Stress Disorder. Edited by Friedman MJ, Charney DS, Deutch AY. Philadelphia, Lippincott-Raven, 1995, pp 203–224Google Scholar
21. Ledoux JE: Setting stress into motion: brain mechanisms of stimulus evaluation. Ibid, pp 125–134Google Scholar
22. Shalev AY, Freedman S, Peri T, Brandes D, Sahar T: Predicting PTSD in trauma survivors: prospective evaluation of self-report and clinician-administered instruments. Br J Psychiatry 1997; 170:558–564Crossref, Medline, Google Scholar
23. Shalev AY, Sahar T, Freedman S, Peri T, Glick N, Brandes D, Orr SP, Pitman RK: A prospective study of heart rate responses following trauma and the subsequent development of posttraumatic stress disorder. Arch Gen Psychiatry 1998; 55:553–559Crossref, Medline, Google Scholar
24. Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, Pitman RK: Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry 1998; 155:630–637Link, Google Scholar
25. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, Keane TM: A clinician rating scale for assessing current and lifetime PTSD: the CAPS-1. Behavior Therapist 1990; 13:187–188Google Scholar
26. Spitzer RL, Williams JBW, Gibbon M, First MB: Instruction Manual for the Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
27. Marmar CR, Weiss DS, Schlenger WE, Fairbank JA, Jordan BK, Kulka RA, Hough RL: Peritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans. Am J Psychiatry 1994; 151:902–907Link, Google Scholar
28. Horowitz MJ, Wilner N, Alvarez W: Impact of Event Scale: a measure of subjective stress. Psychosom Med 1979; 41:209–218Crossref, Medline, Google Scholar
29. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA: Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
30. Keane TM, Caddell JM, Taylor KL: Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. J Consult Clin Psychol 1988; 56:85–90Crossref, Medline, Google Scholar
31. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
32. Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM: Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry 1987; 44:970–975Crossref, Medline, Google Scholar
33. Shalev AY, Orr SP, Pitman RK: Psychophysiologic assessment of traumatic imagery in Israeli civilian patients with posttraumatic stress disorder. Am J Psychiatry 1993; 150:620–624Link, Google Scholar
34. Fridlund AJ, Cacioppo JT: Guidelines for human electromyographic research. Psychophysiology 1986; 23:567–589Crossref, Medline, Google Scholar
35. Ornitz EM, Guthrie D: Long-term habituation and sensitization of the acoustic startle response in the normal adult human. Psychophysiology 1989; 26:166–173Crossref, Medline, Google Scholar
36. Pitman RK, Shalev AY, Orr SP: Post-traumatic stress disorder: emotion, conditioning, and memory, in The Cognitive Neurosciences, 2nd ed. Edited by Gazzaniga MS. Cambridge, Mass, MIT Press, 1999, pp 1133–1147Google Scholar
37. Shalev AY, Peri T, Canneti L, Schreiber S: Predictors of PTSD in injured trauma survivors: a prospective study. Am J Psychiatry 1996; 153:219–225Link, Google Scholar
38. McFarlane AC: Avoidance and intrusion in posttraumatic stress disorder. J Nerv Ment Dis 1992; 180:439–445Crossref, Medline, Google Scholar



