Relationship of Obstetric Complications and Differences in Size of Brain Structures in Monozygotic Twin Pairs Discordant for Schizophrenia
Abstract
OBJECTIVE: The aim of the study was to determine whether a history of obstetric complications and congenital minor physical anomalies are related to differences in the characteristics of brain structures observed within monozygotic twin pairs discordant for schizophrenia. METHOD: The size of the bilateral hippocampi and cerebral ventricles was studied by magnetic resonance imaging in 22 monozygotic twin pairs discordant for schizophrenia. Obstetric complications and minor physical anomalies were independently assessed through parental report and examination, respectively. RESULTS: Compared with the well co-twins, the ill twins consistently had smaller left and right hippocampi as well as larger left lateral ventricles and third ventricles. Relatively small left and right hippocampi were each significantly related to labor-delivery complications and to prolonged labor per se. Relatively large right lateral ventricle size and large total ventricle size were significantly related to labor-delivery complications, prolonged labor, neonatal complications, and total complications for the entire reproductive sequence. In contrast, these brain size differences were not significantly associated with pregnancy complications or minor physical anomalies. CONCLUSIONS: Trauma at the time of labor and delivery and especially prolonged labor appear to be of importance for brain structure anomalies associated with schizophrenia.
An increasing number of studies using postmortem, computerized tomography (CT scan), or magnetic resonance imaging (MRI) techniques have provided evidence for subtle cerebral structural abnormality in individuals with schizophrenia (1–5). The most consistently replicated finding is that of larger lateral ventricles and, somewhat less frequently, a larger third ventricle (2, 6). Studies have also reported enlarged cortical sulci or fissures, suggesting a mild degree of diffuse volume loss (7–11). A smaller frontal lobe area, smaller cerebral and cranial size, and a smaller volume of gray matter in the hippocampus and temporal lobe have also been found (12). Postmortem studies have provided some, but less consistent evidence for subtle structural anomalies primarily in the limbic temporal lobe areas (13), including smaller hippocampal and/or parahippocampal volumes (14, 15); alterations in cell density and cytoarchitectural changes in the hippocampus, frontal and enterorhinal cortex, and cingulate gyrus (16–22); and enlarged temporal horns (23, 24).
However, the absolute size of these reported differences between individuals with schizophrenia and normal comparison subjects has been generally small, with notable overlap between the two groups and considerable normal variation within each group. Such normally occurring interindividual variations may mask subtle, yet potentially informative cerebral characteristics related to schizophrenia. To allow a refined focus on schizophrenia-related cerebral characteristics, researchers have sought a research strategy that would reduce the normal variation in brain structure characteristics across individuals.
Researchers have thus investigated the brain structure characteristics in monozygotic twins in pairs that are discordant for schizophrenia. In such research, any differences in brain structure between the twin with schizophrenia and the genetically identical co-twin without schizophrenia must be considered to reflect the disease and the effect of environmental factors.
Two such studies of discordant monozygotic twin pairs have been conducted to date. A CT study of 12 monozygotic twin pairs discordant for schizophrenia (25) found larger cerebral ventricles in the ill twin than in the well co-twin in 11 of the 12 pairs. A subsequent investigation by Suddath et al. (26) used MRI to study 15 monozygotic pairs discordant for schizophrenia. Compared with the well twins, the ill twins had a smaller left hippocampus (in 14 of 15 pairs) and a smaller right hippocampus (in 13 of 15 pairs), as well as a larger left lateral ventricle (in 14 of 15 pairs), a larger right lateral ventricle (in 13 of 15 pairs), and a larger third ventricle (in 13 of 15 pairs). Additional investigations of discordant monozygotic twins revealed similar within-pair differences in the size of the lateral ventricles and the hippocampi and amygdala (27).
Although the results from the discordant twin pairs clearly indicate that differences in these brain structures are related to schizophrenia and, by deduction, must in some way be the result of environmental influences, important questions remain about what particular environmental factors have caused the differences within pairs and about whether the differences are primary or secondary to the disease (26).
Obstetric complications constitute an early environmental factor that has received considerable attention in association with the subsequent development of schizophrenia (28, 29). McNeil et al. (30) investigated the history of obstetric complications in the cases studied by Suddath et al. (26), as well as in a larger cohort of monozygotic twins studied by Torrey et al. (27), which included discordant, concordant, and normal comparison pairs. Obstetric complications were found with significantly increased frequency in pairs containing one or more twins with schizophrenia (both discordant and concordant pairs) compared with comparison pairs. Complications of labor and delivery were especially characteristic of the discordant twin pairs, and prolonged labor was the most salient specific obstetric complication. The prominence of prolonged labor was similar to findings in samples of individuals with schizophrenia born singly (28, 29, 31).
In contrast, prenatal insults did not consistently characterize these discordant twin pairs. Compared with concordant and normal comparison pairs, the discordant pairs did not have an increased rate of pregnancy complications (30) or of minor physical anomalies (slight somatic defects that putatively develop during early gestation), although the ill twins tended, nonsignificantly, to have more minor physical anomalies than their well co-twins (32). On the other hand, notable within-pair differences were found among discordant twins on dermatoglyphic characteristics that are established during the second trimester of gestation (33, 34). Although those results seem to indicate a prenatal influence disrupting the course of development in individuals with schizophrenia (27), our previous findings in this sample indicated the importance of trauma at the time of labor and delivery and suggested that birth order may be associated with the timing of obstetric complications occurring in the sample. Discordant pairs in which the twin with schizophrenia was born second in the pair had very high rates of prolonged or precipitous labor (88%) but lower rates of pregnancy complications (38%), while the opposite was true for pairs in which the twin with schizophrenia was born first. Birth order is thus also of interest as a possible mediator of a history of obstetric complications in twin pairs discordant for schizophrenia and may have a relationship to brain structure characteristics.
The purpose of the study reported here was to examine the relevance of obstetric complications and minor physical anomalies for the differential sizes of brain structures observed in the twins with schizophrenia in discordant monozygotic pairs. The sample consisted of discordant pairs in which we previously investigated obstetric complications (30) and thus included the smaller subset of cases originally reported by Suddath et al. (26). The study tested the hypothesis that smaller relative size of the right and left hippocampi and larger relative size of the right lateral, left lateral, and third ventricles in the ill twin versus the well twin are related to increased rates of obstetric complications, prolonged labor per se, and minor physical anomalies. Differences in the size of brain structures were also investigated in relation to within-pair birth order. No directional hypothesis was posited for this latter relationship, as birth order was differentially related to pregnancy complications versus labor-delivery complications in this twin sample (30).
METHOD
Subjects
The current sample consisted of 22 monozygotic twin pairs in which one twin had schizophrenia (19 pairs) or schizoaffective disorder (three pairs) and the co-twin was psychiatrically normal (30). Psychiatric diagnoses of the ill twins were determined according to DSM-III-R criteria by using the Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P) (35). The mean severity of illness (axis V of DSM-III-R) during the period just preceding the investigation was 40.1 (SD=13.6). Psychiatric normality in the co-twins was defined as the absence of an axis I or II disorder determined according to DSM-III-R criteria by using the Structured Clinical Interview for DSM-III-R—Non-Patient Version (SCID-NP) (36). Monozygosity was determined by examination of 19 red blood cell antigens and physical appearance. The twins were born in the United States and Canada between 1943 and 1971. Nine of the pairs were female. In 14 of the 22 pairs, the ill twin was the first-born in the pair. Mean age at the time of adult assessment was 30.9 years (SD=6.2). The probability that any of the discordant pairs would become concordant in the future was presumed to be very low, as an average of 14.0 years (range=5–30) had elapsed from illness onset of the proband until the time of the investigation (March 1993); illness onset in a previously normal co-twin is very infrequent after 4 years of discordance (37).
After giving subjects a complete description of the study, we obtained their written informed consent to participate.
MRI Assessment
The MRI assessment procedure has been presented in detail previously (26). A 1.5-T General Electric Signa scanner was used to obtain T1-weighted coronal and sagittal slices and T2-weighted transverse slices with spin-echo pulse sequences. Contiguous, 5.0-mm-thick coronal slices (TR=800 msec, TE=20 msec) were oriented parallel to the floor of the fourth ventricle. A total of 30 slices were taken, beginning at the frontal pole and extending rostrally for 15 cm, thus including most of the occipital lobe.
Pairs of investigators who were blind to the identities of the subjects measured the MRI scans using an off-line computerized image analysis system described elsewhere (26). Values were determined for volumes of total prefrontal and temporal regions, and specific focus was concentrated on six contiguous slices through the temporal lobe. The amygdala, anterior hippocampus, third ventricle, and temporal horns were outlined manually by using a computer-driven cursor. The volumes of these structures and of the lateral ventricles (slices 1–6) were calculated by summing the area for the structure of interest and multiplying by the thickness of the slice. Reliability of measurement was determined by assessing agreement between the two raters’ scores for 48 bilateral measurements of the prefrontal lobe, temporal lobe, and white matter. The interrater reliability was high (intraclass correlation coefficient=0.99 [p<0.001]).
The anterior hippocampus was defined at the rostral-most extent of the hippocampus just caudal to the amygdala slice. The six slices through the anterior temporal lobe included the first slice, which passed through the amygdala, and then the next four slices, through the anterior hippocampus. Thus, the anterior hippocampus region comprised the first 2 cm of the rostral hippocampus. The amygdala and temporal horns were not used in further analyses for two reasons: 1) the search for a relationship between hippocampal volume and obstetric complications was hypothesis driven, based on the relevance of obstetric complications to schizophrenia, and 2) it was observed earlier that the anterior hippocampus was the region that most differentiated the ill from the well twin. The amygdala also was not used in further analyses because it was contained in only one slice and was therefore not reliably measured. The same was true for the temporal horns. The intraclass correlation coefficient for the four sections through the anterior hippocampal formation was 0.82.
For the current analyses, brain structure data were presented as within-monozygotic-pair scores representing the percentage difference within each pair between the twin with schizophrenia and the well co-twin on the structure of interest. The signed percentage within-pair difference was calculated as the size value for the ill twin minus the size value for the well co-twin, divided by the value for the ill twin. Negative scores (percentages) thus resulted when the ill twin had a smaller size value than the well co-twin, and positive scores resulted when the opposite was true. These percentage scores were calculated for the right and left rostral hippocampus, the right and left lateral ventricles, and the third ventricle. For analysis in relation to obstetric complications and minor physical anomalies, we calculated an additional score representing the sum of the three signed (percentage) scores for the ventricles, to yield a measure of the total relative (within-pair) size of the ventricles.
In three cases, rather extreme within-pair scores were obtained for ventricle (but not hippocampal) size, related to conditions that might invalidate analysis of the relationship between obstetric complications and minor physical anomalies and ventricle size. In one pair, the well twin had had pulmonary stenosis, and in another pair, the well twin was found to have hydrocephaly of unknown origin. In the third pair, the ill twin had a history of postnatal head injury. The pairs containing these three cases were thus excluded from the analyses of ventricle size and obstetric complications and minor physical anomalies (19 remaining pairs), to provide a more equitable basis for investigating these relationships. All 22 pairs were included in analyses of hippocampi size.
Each twin was independently assessed for minor physical anomalies (32) by using a modification of the Waldrop scale (38). The Waldrop scale encompasses a total of 19 anomaly items in the head, eye, ear, mouth, hands, and feet. It yields a weighted score summarizing the incidence of minor physical anomalies. The examination is performed quickly and requires very minimal removal of clothing. All examinations were performed by the same examiner (P. Quinn, M.D.), who was totally uninformed of the pair’s history of obstetric complications and results of the MRI examinations. Scores on minor physical anomalies for the individual twins ranged from 2 to 11, and means for the twin pairs ranged from 2.5 to 10.5. Data on minor physical anomalies were missing for one pair.
Assessment of Obstetric Complications
History of obstetric complications for each twin was obtained through detailed, structured interview with the mother and, in one-third of the cases, also with the father, as described previously (30). The obstetric complications identified through interviews with the parents of these discordant pairs, as well as with the parents of twin pairs concordant for schizophrenia and of normal comparison twins, were blindly scored by the first author and a co-worker according to the twin version of the McNeil-Sjöström Scale for Obstetric Complications (30). The instrument is used to rate each obstetric complication on a 6-point severity scale representing the complication’s presumed potential for somatic damage (especially to the central nervous system) in the offspring. Summary scores were calculated for pregnancy complications, labor-delivery complications, neonatal complications, and total obstetric complications for the entire reproductive sequence (the sum of pregnancy complications, labor-delivery complications, and neonatal complications). (Separate summary scores were also available for each trimester of the pregnancy. However, exploratory analyses showed that trimester data would yield results congruent with complications for the entire pregnancy, and only the summary score for the entire pregnancy was used.) The four summary scores represented the number of obstetric complications occurring at a priori-defined severity levels, specifically, at severity level 3 or higher for pregnancy complications and at severity level 4 or higher for the three remaining obstetric complication summary scores. The choice of these cutoff limits was based on our previous analyses of the sample, which showed distinguishing characteristics of obstetric complications for the discordant pairs (30).
Similarly, the set of operational limits for prolonged labor (more than 10 hours for nulliparous women and more than 6.5 hours for primiparous or multiparous women) had been used in our previous analyses of these twin groups (30). By using these operational limits in our previous study, we found an increased rate of prolonged labor in the group of discordant pairs as a whole compared with the groups of concordant and comparison pairs. Before that study, this set of limits distinguishing normal from abnormal length of labor had been established on the basis of optimal delivery norms in our ongoing prospective study of high-risk offspring of women with a history of psychosis (39). In the study reported here, investigators thus compiled and scored information on obstetric complications for each case independently of MRI data and data on minor physical anomalies and without the knowledge of whether neither twin, one twin, or both twins in the pair had schizophrenia.
Design and Statistical Analysis
The frequency with which the ill twin had smaller hippocampi or larger ventricles than the well co-twin was tested by the sign test (40). We had previously observed a high degree of within-pair interdependence in the scores for both obstetric complications and minor physical anomalies (30, 32). Analysis of obstetric complications and minor physical anomalies in relation to MRI thus used the mean scores for obstetric complications or minor physical anomalies for the two individuals in each twin pair, as in our previous studies of this sample (30, 32). To study the relationship between the six MRI measurement variables and obstetric complications or minor physical anomalies, the discordant pairs were assigned to one of the following two subgroups using an approximate median split: 1) pairs showing an especially great abnormality on a particular MRI variable, such as a small hippocampus or large ventricle, in the ill twin versus the well co-twin and 2) pairs showing little difference on a particular MRI variable between the ill twin and the well co-twin or more normality in brain structure characteristics in the ill twin. When the cutoff for subgrouping could not be placed at the absolute median due to an uneven number of cases or tied values at the median, the cutoff was placed to provide the most homogeneous subgroups with respect to the MRI characteristic. Distributions for size of the left and right lateral ventricles (19 pairs) were thus split at nine versus 10 cases and at 10 versus nine cases, respectively. The distribution for the size of the third ventricle was divided into subgroups of 11 versus eight pairs due to a notable discontinuity in the scores at that point. The distribution for the right hippocampus was split evenly at 11 versus 11 cases, while the distribution for the left hippocampus was split at 12 versus 10 pairs due to tied scores surrounding the true median. The two subgroups for each MRI variable were then compared on summary scores for obstetric complications and minor physical anomalies by using the Mann-Whitney-Wilcoxon test for independent groups (40).
The association between birth order or prolonged labor and categorization into the above two subgroups on each MRI variable was examined using Fisher’s exact probability, and odds ratios and 95% confidence intervals (CIs) were calculated. Values for the relative size of brain structures within the pair were also compared for pairs with prolonged labor versus pairs without prolonged labor by using the Mann-Whitney-Wilcoxon test (40). The relationship between the length of labor (hours) and the values for the relative size of brain structures was analyzed using Spearman rank correlation. Statistical significance was defined as p<0.05. As indicated by the hypothesis, one-tailed tests were utilized for all analyses, except those concerning birth order, for which no directional hypothesis was posited.
RESULTS
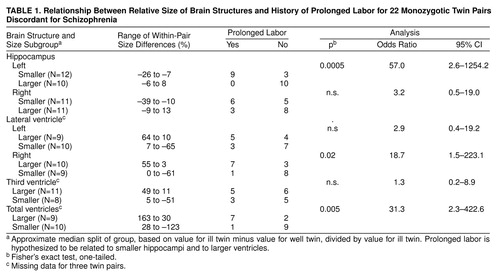
In most of the pairs, the hippocampi were smaller and the ventricles were larger in the ill twin than in the well co-twin. The ill twin had a smaller left hippocampus in 18 of 22 pairs (no tied pairs) (sign test, p=0.002), a smaller right hippocampus in 18 of 22 pairs (one tied pair) (p=0.001), a larger right lateral ventricle in 10 of 19 pairs (two tied pairs) (p=0.32), a larger left ventricle in 14 of 19 pairs (two tied pairs) (p=0.006), and a larger third ventricle in 14 of 19 pairs (no tied pairs) (p=0.03). Nevertheless, for each of the brain areas, the different twin pairs showed a considerable range of scores for relative size in the ill versus well twin, providing an appropriate basis for subgrouping. table 1 shows the ranges of within-pair values in the two subgroups for each MRI variable.
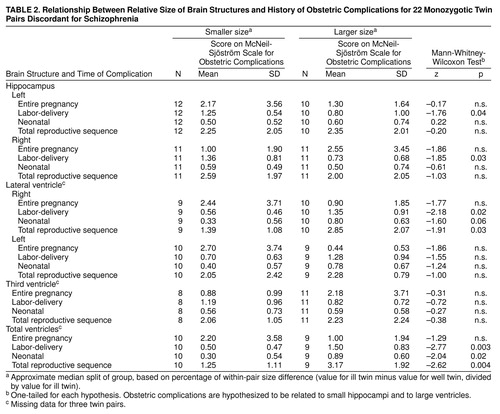
As hypothesized, rates of obstetric complications were significantly higher for the subgroups of twin pairs in which the ill twin had especially small hippocampi and especially large ventricles, and the relationships were specific for both brain area and timing of the obstetric complications (table 2). An especially small left hippocampus and a small right hippocampus in the ill twin were each significantly related to increased rates of labor-delivery complications only. Furthermore, an especially large right lateral ventricle and an especially large total ventricle size in the ill twin were each significantly related to increased rates of labor-delivery complications, neonatal complications, and total obstetric complications for the entire reproductive sequence. An especially large left lateral ventricle and a larger third ventricle in the ill twin were not associated with an increase in any obstetric complication summary score.
As also hypothesized, the occurrence of prolonged labor was highly significantly related to smaller left hippocampus size in the ill twin and also significantly related to larger right lateral ventricle size and larger total ventricle size in the ill twin (table 1). Prolonged labor occurred frequently, in nine of 12 twin pairs, in the subgroup in which the ill twin had a relatively small left hippocampus and did not occur at all in the subgroup in which the ill twin had a relatively larger left hippocampus.
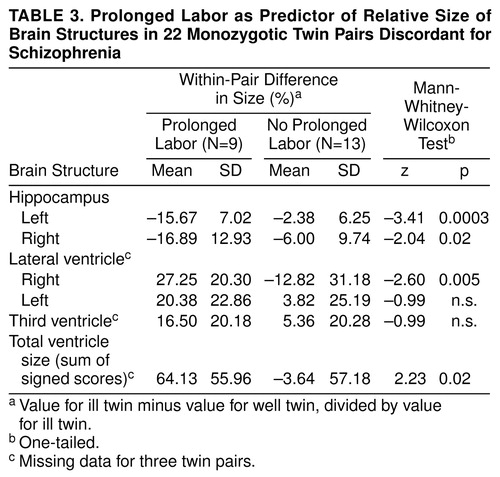
When prolonged labor was used as a predictor variable for relative size of the brain structures within the pair, pairs with prolonged labor, compared with the remaining pairs, were found to have significantly smaller left and right hippocampus size in the ill twin and also significantly larger right lateral ventricle size and larger total ventricle size in the ill twin (table 3). Furthermore, length of labor, measured in hours, correlated significantly with relatively small left hippocampus size (Spearman rank correlation rs=–0.55, N=20, p=0.006), larger right lateral ventricle size (rs=0.53, N=17, p=0.02), and larger total ventricle size in the ill twin (rs=0.41, N=17, p=0.05), but not with relatively small right hippocampus size (rs=–0.28, N=20, n.s.), larger left lateral ventricle size (rs=0.01, N=17, n.s.), or larger third ventricle size (rs=0.17, N=17, n.s.) in the ill twin.
In contrast, the signs of brain abnormality in the ill twin, such as especially small hippocampi and especially large ventricles, were not significantly related to rates of pregnancy complications (table 2) or to rates of minor physical anomalies (table 4). Furthermore, birth order in itself was not significantly related to small hippocampi or large ventricles in the ill twin versus the well twin (results available from authors on request).
DISCUSSION
Within 22 monozygotic twin pairs discordant for schizophrenia, the ill twin significantly more often had smaller left and right hippocampi, a larger left lateral ventricle, and a larger total ventricle size than the mentally well co-twin. These results were congruent with the results for a smaller subsample of these cases that had been previously examined (26). The results also corroborate previous findings in studies of individuals with schizophrenia born singly (1, 6, 14).
As predicted, obstetric complications were found to be significantly associated with the relative size of brain structures of the ill twins. Increased rates of total obstetric complications for the entire reproductive sequence, as well as of labor-delivery and neonatal complications, were associated with relatively large right ventricle size and relatively large total ventricle size in the ill twin. Labor-delivery complications were more specifically related to relatively small left and right hippocampus size in the ill twin. As a single obstetric complication chosen a priori, prolonged labor showed an impressively strong relationship to smaller hippocampal size and larger right lateral ventricle and total ventricle size in the ill twin. Prolonged labor occurred very frequently, in nine of 12 pairs, and only in pairs in which the ill twin had a notably smaller left hippocampus. The left hippocampus has been identified as a brain area of particular importance in schizophrenia (6), especially in relation to positive psychotic symptoms (2). Indeed, correlational analyses showed increased length of labor to be significantly related to smaller relative size of the left hippocampus in the ill twin versus the well twin, as well as to larger right lateral ventricle and total ventricle size in the ill twin versus the well twin.
Prolonged labor thus bore an impressive association with the relative brain structure characteristics in the ill twins. This obstetric complication has been found to characterize patients with schizophrenia in previous studies (28, 29, 31). The definition of prolonged labor used in this study (more than 10 hours in null parae, more than 6.5 hours in others) was based on modern Swedish recommendations for optimal delivery times and was previously used empirically in our prospective study of high-risk offspring (39, 41) and in our previous study of obstetric complications in the these twin pairs (30). In our previous study, the discordant pairs had significantly increased rates of prolonged labor defined in this manner compared with normal or concordant monozygotic twin pairs. Thus, prolonged labor was a salient characteristic of the discordant twin group. This definition of prolonged labor is liberal by other obstetric standards; our international obstetric complication scale (42) defines prolonged labor as more than 16 hours and more than 10 hours for null versus one or more parae, respectively. During labor, the mothers of the twins in this study appeared to have been subject to considerable clinical surveillance and assisted with interventions as needed, and thus their labors may not have been extremely protracted, as is sometimes seen in other samples of offspring with schizophrenia. Nevertheless, the significant correlations between actual length of labor and both smaller left hippocampus size and larger left lateral ventricle size and larger total ventricle size indicate the apparent relevance of prolonged labor even within the range of labor durations observed for this sample.
Despite the unusual research opportunities offered by this sample, sample size was nevertheless limited, and the power to detect significant differences in within-group analyses of 22 cases was necessarily limited. The odds ratios for some of the statistically nonsignificant findings in table 1 suggested interesting tendencies for prolonged labor to be associated with the size of other brain structures (right hippocampus odds ratio=3.2; left lateral ventricle odds ratio=2.9), and these associations might have reached statistical significance had the sample size been larger. The small sample size suggests caution in drawing any conclusions about the specificity of relationships between prolonged labor and only the left hippocampus as well as only the right lateral ventricle.
Because of the general absence of medical obstetric records for this North American sample, retrospective maternal reports were used as the source of information on obstetric complications. The validity of maternal reports of obstetric complications has been the subject of much speculation, generally unaccompanied by empirical facts. The first systematic study of psychiatric samples clearly suggested maternal reports to be a satisfactory source of information on obstetric complications (43). In our recent study (44) of medical record information versus retrospective reports of Swedish mothers (mean age=64 years) of patients with schizophrenia and of normal comparison subjects, considerable discrepancies between the records and the reports of obstetric events and conditions 35 years in the past were found for both maternal groups. In both groups, errors of omission were more frequent than errors of commission, with patients’ mothers tending toward more omissions than comparison mothers (Mann-Whitney-Wilcoxon test, N=45 patient mothers and N=34 comparison mothers, p=0.06, two-tailed). In the sample of twins included in this study, evidence indicated clearly that the reports given by the mothers appeared to be free of commissive errors (30). Most reported obstetric complications concerned both the ill and the well twin in the pair, and the well twin was often reported to be the more abnormal of the two when differences in obstetric complications did occur within the pair. Furthermore, the report data had predictive validity, as they strongly related to other characteristics of the twins in adulthood; the obstetric complications reported by parents related significantly to independently assessed minor physical anomalies (32), neurological abnormality (45), and brain structure size. These three correlates of obstetric complications can all be objectively and reliably investigated (26, 46, 47), while the reliability of information on obstetric complications is less certain. Given the possible general tendency toward errors of omission (but not commission) in data on obstetric complications, the possibility exists that even more striking relationships would have been observed between obstetric complications and brain structure characteristics if information on the obstetric complications been entirely veridical.
In total, the results for pregnancy complications, minor physical anomalies, labor-delivery complications, birth order, and brain structure characteristics suggest at least two possible trajectories, with seemingly different temporal paths for obstetric complications within the twin sample (30). One trajectory relates labor-delivery complications to small left hippocampus size to later schizophrenia. Evidence for this trajectory includes our finding that brain abnormality in the ill twin (small hippocampus, enlarged ventricles) was significantly positively related to total obstetric complications and especially to labor-delivery complications, but not to prenatal influence as represented by both pregnancy complications and minor physical anomalies. This relationship may be partly mediated by birth order, as discordant pairs in which the twin with schizophrenia was born second in the pair had very high rates of labor-delivery complications but lower rates of pregnancy complications (30), and increased rates of labor-delivery complications were related to both small hippocampi and enlarged ventricles in the ill twin. Second-born birth order in the ill twin thus appears to play a role by mediating labor-delivery complications.
The other possible trajectory relates pregnancy complications to minor physical anomalies to nondeviant brain structure (in the ill versus the well co-twin) to later schizophrenia. This trajectory may tend especially to characterize pairs in which the twin with schizophrenia was first born (30). This subgroup had high rates of pregnancy complications (73%) and lower rates of prolonged or precipitous labor (33%) (30). Pregnancy complications were significantly positively related to minor physical anomalies in these discordant pairs (32), but neither pregnancy complications nor minor physical anomalies were significantly related to structural brain abnormality in the ill (versus the well) discordant twins. In fact, both pregnancy complications and minor physical anomalies tended to be related to less brain abnormality in the ill twin (table 2: right hippocampus and right and left lateral ventricles; table 4: all brain structures). The most likely explanation for this pattern of results is that the brain regions found to be affected in these ill twins are susceptible to late-occurring obstetric complications, such as labor-delivery complications and even neonatal complications (table 2), but not to the earlier developmental events represented by minor physical anomalies and pregnancy complications. Furthermore, minor physical anomalies and pregnancy complications were significantly negatively related to labor-delivery complications in this sample (48), supporting the concept of two different developmental trajectories.
With respect to possible etiological variability, there may well be different timing sequences for obstetric complications as well as different etiological influences among the twin pairs. Twins with schizophrenia whose structural brain characteristics do not differ notably from those of their well co-twins may have received their illness through a different etiological process than did ill twins with notable brain differences, which seem to be related to obstetric complications, and to labor-delivery complications in particular. The concordant pairs in the twin series studied by Torrey et al. are interesting in this respect, as our previous study found that they had significantly lower rates of total labor-delivery complications than the discordant pairs (Mann-Whitney-Wilcoxon test, N=10 and N=23, p=0.02), and only one of the 10 concordant pairs had an abnormal length of labor compared with 52% of the discordant pairs (30). Although the classic study of Fischer (49) indicated that genetic factors related to schizophrenia are relevant for discordant pairs, some authors have suggested that discordant pairs represent proportionally more “environmental” etiological influence, while concordant pairs represent more “genetic” etiological influence (50–52). This suggestion would be congruent with the significantly increased rates of labor-delivery complications in discordant (versus concordant) twin pairs as well as with the apparent relevance of labor-delivery complications for especially notable differences in brain structure size within discordant pairs.
Interestingly, both the ill and the well co-twins in this study had experienced the same prolonged labor and many of the other obstetric complications as well, but with entirely different psychiatric outcomes. Several possible explanations for this apparent discrepancy in outcome exist. First, the two twins may in reality have received different exposures to what operationally appears to be the same complication. Previous evidence has suggested clearly that only one of the two monozygotic twins in a pair may be compromised by seemingly untoward prenatal or perinatal influences (30) and that the effect on individuals may depend on many varied and differential prenatal or perinatal intra-uterine influences. For example, twins in the same pair may experience very different prenatal environments as a function of inequality of blood flow, resulting in a wide range of developmental deviations, from poorly perfused cranial tissue and abnormal tissue formation to gross structural deviations, growth retardation with large within-pair birth weight differences, and even death (53, 54). Differential blood flow could result not only in the more obvious within-pair differences in provision of oxygen and other nutrients but also in potential differences in exposure to infectious and teratogenic agents during pregnancy and labor. The observed within-pair differences in brain structure size and associated psychiatric outcome would be congruent with such variations in exposure to the early perinatal events recorded for these discordant twin pairs.
Second, the obstetric complications that occurred in these discordant pairs may possibly have had a deleterious effect on the well co-twins but did not lead to schizophrenia for other unknown reasons. A significantly increased rate of neurological abnormality was observed in these well co-twins compared with comparison twins (45), and their neurological abnormality was found to be significantly related to obstetric complications (45). Lewis et al. (55) have raised the possibility that early trauma resulting, for example, from labor-delivery complications might actually reduce rather than increase risk for subsequent schizophrenia. Such a reduced risk could possibly be a result of reorganization of the surviving neural circuitry (56), leading to other neurological abnormality but not to schizophrenia.
Third, other influences occurring during subsequent developmental phases could have provided the basis for divergence in both brain development and mental health status. Perinatal trauma such as that observed in the current sample is known to be related to considerable individual variation in long-term damage and recovery over time (57) as a result of such influences. The results presented here represent only the relationship between obstetric complications and adult status regarding brain structure size and the presence or absence of schizophrenia and in no way take into account environmental influences occurring after the neonatal period.
The possibility that labor-delivery complications in individuals who later develop schizophrenia are secondary consequences of preexistent abnormality in the fetus has been raised by a number of authors (58–60). This possibility would not explain the current findings, as it would be unlikely that a second-born twin who later developed schizophrenia would effectively cause a prolonged labor, while a first-born twin who later developed schizophrenia or two such twins in the same pair (concordants) would not cause prolonged labors. Second-born status is known to be associated with increased risk for asphyxia and other labor-delivery complications (61). Furthermore, our analyses of data on obstetric complications in the current twin sample, in individuals with schizophrenia born singly, and in genetically high-risk offspring indicated clearly that fetuses with putative prenatal abnormality do not have increased rates of labor-delivery complications, including prolonged labor (41, 62). The current results showing brain structure abnormality in the ill twin to be positively related to labor-delivery complications but not related to both prenatal factors (pregnancy complications and minor physical anomalies) would further support the conclusion that labor-delivery complications are not secondary consequences of an identifiable preexistent fetal abnormality. Had prenatal abnormality led to labor-delivery complications, then we should have observed brain abnormality to be related to both prenatal factors and labor-delivery complications, with prenatal factors and labor-delivery complications positively related to each other. Neither of these conditions obtained. Nevertheless, we cannot rule out that the labor-delivery complications reflect a primary developmental defect in the fetus rather than an independent trauma to the fetus at delivery. Indeed a recent study has demonstrated that even gross cerebral malformations can be associated with prenatal complications (63). One additional caveat here, in keeping with findings on the tendency for omissions in obstetrical histories, is that relevant adverse prenatal events may be less dramatic and concrete and thus less likely to be recalled and rated than are labor and delivery events.
The present findings of a relationship between obstetric complications and brain structure abnormalities among discordant pairs do not per se indicate that labor-delivery complications “cause” schizophrenia, as the current within-group analyses only compared obstetric complication history for twins with schizophrenia who had more deviant versus less deviant brain structure characteristics. What the current findings do suggest is that obstetric complications, especially labor-delivery complications, and very specifically prolonged labor are related to brain structure characteristics that are a central focus in schizophrenia. These findings are all the more salient, as they emanate from the very powerful discordant-twin research design and implicate early environmental factors that have been repeatedly observed in other studies of individuals with schizophrenia (28, 29).
Important insights into the development of schizophrenia might be obtained by providing a qualified answer to the question of why only one of two genetically identical twins, who are seemingly exposed to the very same prolonged labor, develops both schizophrenia and apparently associated structural brain changes lasting into adulthood.
Received Nov. 18, 1998; revision received June 23, 1999; accepted June 30, 1999. From the Section for Epidemiology, Department of Community Medicine, Lund University, Malmö University Hospital, Malmö, Sweden; and the Clinical Brain Disorders Branch, NIMH, Bethesda, Md. Address reprint requests to Dr. McNeil, Department of Community Medicine, University Hospital MAS, S-205 02 Malmö, Sweden; [email protected] (e-mail). Supported by grant 3793 from the Swedish Medical Research Council; the Faculty of Medicine, Lund University; NIMH grant MH-41176; the Söderström-König Foundation, Sweden; and the Theodore and Vada Stanley Foundation, USA. The authors thank E. Fuller Torrey, M.D., for providing access to project data, and acknowledge the contributions of Patricia Quinn, M.D., Richard L. Suddath, M.D., and Irving I. Gottesman, Ph.D., to conducting the twin project.
 |
 |
 |
 |
1. Bogerts B: Recent advances in the neuropathology of schizophrenia. Schizophr Bull 1993; 19:431–445Crossref, Medline, Google Scholar
2. Bogerts B: The temporolimbic system theory of positive schizophrenic symptoms. Schizophr Bull 1997; 23:423–435Crossref, Medline, Google Scholar
3. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyrus volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Google Scholar
4. Degreef G, Ashtari M, Bogerts B, Bilder RM, Jody DN, Alvir JMJ, Lieberman JA: Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Arch Gen Psychiatry 1992; 49:531–537Crossref, Medline, Google Scholar
5. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
6. Crow TJ, Johnstone EC: Schizophrenia: nature of the disease process and its biological correlates, in Handbook of Physiology—The Nervous System, V. Edited by Plum F. Baltimore, American Physiological Society, 1987, pp 843–869Google Scholar
7. Shelton RC, Weinberger DR: X-ray computerized tomography studies in schizophrenia: a review and synthesis, in Handbook of Schizophrenia, vol 1: the Neurology of Schizophrenia. Edited by Nasrallah HA, Weinberger DR. Amsterdam, Elsevier, 1986, pp 207–250Google Scholar
8. Pakkenberg B: Post-mortem study of chronic schizophrenic brains. Br J Psychiatry 1987; 151:744–752Crossref, Medline, Google Scholar
9. Cannon TD, Mednick SA: Fetal neural development and adult schizophrenia: an elaboration of the paradigm, in Fetal Neural Development and Adult Schizophrenia. Edited by Mednick S, Cannon TD, Barr CE, Lyon M. Cambridge, UK, Cambridge University Press, 1991Google Scholar
10. Andreasen NC, Swayze VW II, Flaum M, Yates WR, Arndt S, McChesney C: Ventricular enlargement in schizophrenia evaluated with computed tomographic scanning: effects of gender, age, and stage of illness. Arch Gen Psychiatry 1990; 47:1008–1015Google Scholar
11. Gur RE, Mozley D, Resnick SM: Magnetic resonance imaging in schizophrenia. Arch Gen Psychiatry 1991; 48:407–412Crossref, Medline, Google Scholar
12. Chua SE, McKenna PJ: Schizophrenia—a brain disease? a critical review of structural and functional cerebral abnormality in the disorder. Br J Psychiatry 1995; 166:563–582Crossref, Medline, Google Scholar
13. Weinberger DR: Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry 1999; 45:395–402Crossref, Medline, Google Scholar
14. Bogerts B: Zur Neuropathologie der Schizophrenien. Fortschr Neurol Psychiatr 1984; 52:428–437Crossref, Medline, Google Scholar
15. Altshuler LL, Casanova MF, Goldberg TF, Kleinman JE: The hippocampus and parahippocampus in schizophrenic, suicide, and control brains. Arch Gen Psychiatry 1990; 47:1029–1034Google Scholar
16. Kovelman JA, Scheibel AB: A neurohistological correlate of schizophrenia. Biol Psychiatry 1984; 19:1601–1621Google Scholar
17. Jakob J, Beckmann H: Prenatal development disturbances in the limbic allocortex in schizophrenics. J Neural Transm 1986; 65:303–326Crossref, Medline, Google Scholar
18. Falkai P, Bogerts B: Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986; 236:154–161Crossref, Medline, Google Scholar
19. Benes FM: An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Arch Gen Psychiatry 1987; 44:608–616Crossref, Medline, Google Scholar
20. Conrad AJ, Abebe T, Austin R, Forsythe S, Scheibel AB: Hippocampal cell disarray in schizophrenia. Arch Gen Psychiatry 1991; 48:413–417Crossref, Medline, Google Scholar
21. Akbarian S, Bunney WE, Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG: Altered distribution of nicotinamide-adenine-dinucleotide phosphate-diaphorase cells in frontal cortex of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry 1993; 50:169–177Crossref, Medline, Google Scholar
22. Akbarian S, Viñela A, Kim JJ, Potkin SG, Bunney WE, Jones EG: Distorted distribution of nicotinamide-adenine-dinucleotide phosphate, diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry 1993; 50:178–187Crossref, Medline, Google Scholar
23. Brown R, Colter N, Corsellis J: Postmortem evidence of structural brain changes in schizophrenia: differences in brain weight, temporal horn area and parahippocampal gyrus compared with affective disorder. Arch Gen Psychiatry 1986; 43:36–42Crossref, Medline, Google Scholar
24. Crow TJ, Ball J, Bloom SR: Schizophrenia as an anomaly of development of cerebral asymmetry. Arch Gen Psychiatry 1989; 46:1145–1150Google Scholar
25. Reveley AM, Reveley MA, Clifford CA, Murray RM: Cerebral ventricular size in twins discordant for schizophrenia. Lancet 1982; 1:540–541Crossref, Medline, Google Scholar
26. Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR: Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 1990; 322:789–794Crossref, Medline, Google Scholar
27. Torrey EF, Bowler AE, Taylor EH, Gottesman II: Schizophrenia and Manic Depressive Disorder: The Biological Roots of Mental Illness as Revealed by a Landmark Study of Identical Twins. New York, Basic Books, 1994Google Scholar
28. McNeil TF: Obstetric complications, in Neurodevelopmental Basis of Schizophrenia. Edited by Waddington JL, Buckley PF. Austin, Tex, RG Landes, 1995, pp. 62–78Google Scholar
29. McNeil TF: Perinatal risk factors and schizophrenia: selective review and methodological concerns. Epidemiol Rev 1995; 17:107–112Crossref, Medline, Google Scholar
30. McNeil TF, Cantor-Graae E, Torrey EF, Sjöström K, Bowler A, Taylor E, Rawlings R, Higgins ES: Obstetric complications in histories of monozygotic twins discordant and concordant for schizophrenia. Acta Psychiatr Scand 1994; 89:196–204Crossref, Medline, Google Scholar
31. McNeil TF: Obstetric factors and perinatal injuries, in Handbook of Schizophrenia, Vol 3: Nosology, Epidemiology and Genetics. Edited by Tsuang MT, Simpson JC. Amsterdam, Elsevier, 1988, pp 319–344Google Scholar
32. Cantor-Graae E, McNeil TF, Torrey EF, Quinn P, Bowler A, Sjöström K, Rawlings R: Link between pregnancy complications and minor physical anomalies in monozygotic twins discordant for schizophrenia. Am J Psychiatry 1994; 115:1188–1193Google Scholar
33. Bracha HS, Torrey EF, Bigelow LB, Lohr JB, Linington BB: Subtle signs of prenatal maldevelopment of the hand ectoderm in schizophrenia: a preliminary monozygotic twin study. Biol Psychiatry 1991; 30:719–725Crossref, Medline, Google Scholar
34. Bracha HS, Torrey EF, Gottesman II, Bigelow LB, Cunniff C: Second-trimester markers of fetal size in schizophrenia: a study of monozygotic twins. Am J Psychiatry 1992; 149:1355–1361Google Scholar
35. Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1986Google Scholar
36. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Version (SCID-NP). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
37. Belmaker R, Pollin W, Wyatt RJ, Cohen S: A follow-up of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry 1974; 30:219–222Crossref, Medline, Google Scholar
38. Waldrop MF, Petersen FA, Bell RQ: Minor physical anomalies and behavior in preschool children. Child Dev 1968; 39:391–400Crossref, Medline, Google Scholar
39. McNeil TF, Kaij L: Swedish high-risk study: sample characteristics at age 6. Schizophr Bull 1987; 13:373–381Crossref, Medline, Google Scholar
40. Siegel S, Castellan NJ: Nonparametric Statistics for the Behavioral Sciences. New York, McGraw-Hill, 1988Google Scholar
41. McNeil TF, Blennow G: A prospective study of postpartum psychoses in a high-risk group, 6: relationship to birth complications and neonatal abnormality. Acta Psychiatr Scand 1988; 78:478–484Crossref, Medline, Google Scholar
42. McNeil TF, Sjöström K: McNeil-Sjöström Scale for Obstetric Complications. Malmö, Sweden, Lund University Department of Psychiatry, 1995Google Scholar
43. O’Callaghan E, Larkin C, Waddington JL: Obstetric complications in schizophrenia and the validity of maternal recall. Psychol Med 1990; 20:89–94Crossref, Medline, Google Scholar
44. Cantor-Graae E, Cardenal S, Ismail B, McNeil TF: Recall of obstetric events by mothers of schizophrenic patients. Psychol Med 1998; 28:1239–1243Google Scholar
45. Cantor-Graae E, McNeil TF, Rickler KC, Sjöström K, Rawlings R, Higgins ES, Hyde TM: Are neurological abnormalities in well discordant monozygotic co-twins of schizophrenic subjects the result of perinatal trauma? Am J Psychiatry 1994; 151:1194–1199Google Scholar
46. Ismail B, Cantor-Graae E, McNeil TF: Minor physical anomalies in schizophrenic patients and their siblings. Am J Psychiatry 1998; 155:1695–1702Google Scholar
47. Ismail B, Cantor-Graae E, McNeil TF: Neurological abnormalities in schizophrenic patients and their siblings. Am J Psychiatry 1998; 155:84–89Link, Google Scholar
48. McNeil TF, Cantor-Graae E: Does preexisting abnormality cause labor-delivery complications in fetuses who will develop schizophrenia? Schizophr Bull 1999; 25:425–435Google Scholar
49. Fischer M: Genetic and environmental factors in schizophrenia. Acta Psychiatr Scand 1973; 238:1–153Google Scholar
50. Gottesman II, Shields J: Schizophrenia and Genetics: A Twin Study Vantage Point. New York, Academic Press, 1972Google Scholar
51. Markow TA, Gottesman I: Fluctuating dermatoglyphic asymmetry in psychotic twins. Psychiatr Res 1989; 29:37–43Crossref, Medline, Google Scholar
52. Lewis S, Chitkara B, Reveley AM, Murray RM: Family history and birthweight in monozygotic twins concordant and discordant for psychosis. Acta Genet Med Gemellol (Roma) 1987; 36:267–273Crossref, Medline, Google Scholar
53. Potter EL: Twin zygosity and placental form in relation to the outcome of pregnancy. Am J Obstet Gynecol 1963; 87:566–577Crossref, Medline, Google Scholar
54. Schinzel AGL, Smith DW, Miller JR: Monozygtic twinning and structural defects. J Pediatr 1979; 95:921–930Crossref, Medline, Google Scholar
55. Lewis SW, Harvey I, Ron M, Murray RM, Reveley A: Can brain damage protect against schizophrenia? a case report of twins. Br J Psychiatry 1990; 157:600–603Crossref, Medline, Google Scholar
56. Steward O: Lesion-inducted neuroplasticity and the sparing or recovery of function following early brain damage, in Early Brain Damage, vol 1: Research Orientations and Clinical Observations. Edited by Almli CR, Finger S. New York, Academic Press, 1984, pp 59–77Google Scholar
57. Huisjes HJ, Touwen BCL, Hoekstra J, van Woerden-Blanksma JT, Bierman-van Eendenburg ME, Jurgens-van der Zee AD, Fidler VJ, Olinga AA: Obstetrical-neonatal neurological relationship: a replication study. Eur J Obstet Gynecol Reprod Biol 1980; 10:247–256Crossref, Medline, Google Scholar
58. Goodman R: Are complications of pregnancy and birth causes of schizophrenia? Dev Med Child Neurol 1988; 30:391–406Google Scholar
59. O’Callaghan E, Gibson T, Colohan HA, Buckley P, Walshe DG, Larkin C, Waddington JL: Risk of schizophrenia in adults born after obstetric complications and their association with early onset of illness: a controlled study. Br Med J 1992; 305:1256–1259Google Scholar
60. Günther-Genta F, Bovet P, Hohlfeld P: Obstetric complications and schizophrenia: a case-control study. Br J Psychiatry 1994; 164:165–170Crossref, Medline, Google Scholar
61. Bryan E: Twins and Higher Multiple Births: A Guide to Their Nature and Nurture. London, Edward Arnold, 1992Google Scholar
62. McNeil TF, Cantor-Graae E, Blennow G: Do “clumsy” fetuses cause labor-delivery complications? a study of offspring at risk for psychosis. Schizophr Res 1996; 22:85–88Crossref, Medline, Google Scholar
63. Vesce F, Farina A, Giorgetti M, Jorizzo G, Bianciotto A, Calabrese O, Mollica G: Increased incidence of preeclampsia in pregnancies complicated by fetal malformation. Gynecol Obstet Invest 1997; 44:107–111Crossref, Medline, Google Scholar



