Ionotropic Glutamate Receptor Binding and Subunit mRNA Expression in Thalamic Nuclei in Schizophrenia
Abstract
OBJECTIVE: Both thalamic and glutamatergic dysfunction have been implicated in the pathophysiology of schizophrenia. The authors examined ionotropic glutamate receptor expression in postmortem samples from patients with schizophrenia and comparison subjects, using the hypothesis that glutamate receptor expression differs in limbic nuclei of the thalamus in schizophrenia. METHOD: N-Methyl-d-aspartate (NMDA), AMPA, and kainate receptor expression was determined in six thalamic nuclei from 12 subjects with DSM-III-R diagnoses of schizophrenia and eight psychiatrically normal individuals. The authors used in situ hybridization to determine NMDAR1, NMDAR2A–NMDAR2D, gluR1–gluR7, KA1, and KA2 subunit mRNA levels and receptor autoradiography to determine binding to glutamate binding sites of the three receptor subtypes and to the glycine, polyamine, and ion channel binding sites of the NMDA receptor. RESULTS: Glutamate receptor expression was lower at both transcriptional (NMDAR1, NMDAR2B, NMDAR2C, gluR1, gluR3, and KA2 subunit mRNAs) and posttranscriptional ([3H]ifenprodil and [3H]MDL105,519 binding to polyamine and glycine sites of the NMDA receptor) levels in the thalamus in patients with schizophrenia than in comparison subjects, but differences were most prominent in nuclei with reciprocal projections to limbic regions. CONCLUSIONS: Abnormalities in NMDA, AMPA, and kainate receptor expression in limbic thalamus are suggestive of the NMDA receptor hypoactivity hypothesis of schizophrenia and are consistent with diminished glutamatergic activity in the thalamus in schizophrenia. Alternatively, these results could suggest abnormal glutamatergic innervation in afferent and/or efferent regions, which are limbic structures that have been implicated in this illness. These results may provide a neurochemical anatomical substrate for antipsychotic therapies targeting ionotropic glutamate receptors.
The critical role of the thalamus in sensory processing, and the rich, reciprocal limbic projections with the frontal and cingulate cortex, hippocampus, nucleus accumbens, and amygdala, has led to speculation that this structure may be dysfunctional in schizophrenia (1). Structural and functional pathology have been detected in the thalamus in schizophrenia, which is consistent with this hypothesis. Lower thalamic cell numbers and volume have been reported in some but not all studies in schizophrenia, in relation to comparison subjects, while lower levels of thalamic metabolism and suggestions of different corticothalamic connectivity have been consistently reported (2–13). However, it is currently unclear which neurochemical substrates are associated with these abnormalities. The glutamatergic system represents a likely candidate, both because most thalamic afferents and efferents are glutamatergic (1, 14, 15) and because pharmacological evidence implicates glutamatergic dysfunction in schizophrenia.
N-Methyl-d-aspartate (NMDA), AMPA, kainate, and metabotropic receptors make up the four families of glutamate receptors, and all are expressed in the thalamus (16, 17). NMDA receptor abnormalities are most often associated with schizophrenia, because the NMDA receptor antagonists phencyclidine (PCP) and ketamine can induce schizophreniform psychosis in normal volunteers and exacerbate psychotic symptoms in schizophrenia patients (18–22). Furthermore, adjunct treatment with conventional antipsychotics of agonists and partial agonists of the glycine coagonist site of the NMDA receptor has been reported in some studies to ameliorate negative psychotic symptoms (23–28). These data have been interpreted to suggest that NMDA receptor hypoactivity is associated with schizophrenia. However, it is not apparent whether a difference in NMDA receptor activity results from a primary defect in NMDA receptors or from dysfunction in one of the other three glutamate receptor families that may secondarily lead to low levels of NMDA receptor activity. Activation of presynaptic kainate receptors facilitates glutamate release and/or decreases GABA(γ-aminobutyric acid)ergic activity, creating a functional interface with postsynaptic NMDA receptors (29–36). Furthermore, the NMDA receptor ion channel is blocked by physiological concentrations of magnesium ions, and partial depolarization of the cell membrane is required to extrude magnesium and allow ion flow though the NMDA receptor channel. Activation of AMPA receptors appears to provide this permissive function, and AMPA receptors are extensively colocalized with NMDA receptors at glutamatergic terminals (37). Finally, there are pre- and postsynaptic metabotropic receptors that also affect NMDA receptor-mediated neurotransmission (38–40). Dysfunction of any of the four glutamate receptors could mimic abnormal NMDA receptor activity.
Both thalamic and glutamatergic dysfunction have been separately associated with schizophrenia, but there are few studies examining thalamic glutamate receptor expression in this illness. Therefore, we measured ionotropic glutamate receptor subunit mRNA levels by in situ hybridization, and receptor binding by receptor autoradiography, in discrete thalamic nuclei in patients with schizophrenia and comparison subjects. The topographic organization of the thalamus allowed us to compare glutamate receptor expression in the limbic nuclei (dorsomedial, anterior, laterodorsal, and central medial) with nonlimbic nuclei (reticular and ventral). Our overall hypothesis was that glutamate receptor expression differs in the limbic thalamic nuclei in schizophrenia.
Method
Subjects
Twelve subjects with schizophrenia and eight nonpsychiatrically ill individuals were studied (Table 1). Subjects were from the Mount Sinai Medical Center Brain Bank. Patients were classified as having schizophrenia if 1) the presence of schizophrenic symptoms could be documented before age 40; 2) the medical records contained evidence of psychotic symptoms and at least 10 years of psychiatric hospitalization with a diagnosis of schizophrenia; 3) the DSM-III-R diagnosis of schizophrenia was agreed on by two experienced clinicians; and 4) neuropathological examination did not reveal Alzheimer’s disease or other degenerative disorders. Neither age (t=1.59, df=18, p=0.13), postmortem interval (t=1.08, df=18, p=0.29), nor sex distribution (χ2=1.02, df=1, p=0.31) were significantly different between the two groups.
The brains obtained at autopsy were prepared by slicing one hemisphere into 1-cm coronal slabs that were immediately frozen on dry ice. Blocks containing the thalamus (four from the left hemisphere and 16 from the right hemisphere) were cryostat-sectioned (20 μm thick), thaw-mounted onto poly-l-lysine-subbed microscope slides, dried, and stored at –80°C until use. The subunits expressing mRNA encoding of NMDA, AMPA, and kainate receptors were investigated by in situ hybridization, and the distribution of ionotropic receptor binding sites was studied by using receptor autoradiography.
Riboprobe in Situ Hybridization
The AMPA receptor subunits are derived from a family of four genes termed gluR1–gluR4, while kainate receptor subunits are derived from genes for the low-affinity gluR5–gluR7 and high-affinity KA1 and KA2 subunits (16). The NMDA receptor subunits are encoded by five genes termed NMDAR1 and NMDAR2A–NMDAR2D (16). NMDAR1 is expressed as one of eight isoforms because of the alternative splicing of exons 5, 21, and 22 (41, 42); our probe recognizes all eight isoforms (Figure 1). Riboprobes were synthesized from linearized plasmid DNA containing subclones of these ionotropic glutamate receptor subunits, as has been previously described (43, 44). In addition, a 743 base pair subclone of the entire coding region of the ubiquitously expressed prolyl isomerase cyclophilin was used for in situ hybridization in the same subjects (45, 46). Two slides per subject for each probe were removed from –80°C storage and fixed in 4% (weight/volume) formaldehyde at room temperature for 1 hour. Slides were processed for in situ hybridization, as we have previously reported (43, 44, 46). After in situ hybridization, slides were apposed to Kodak (Rochester, N.Y.) BioMax MR1 film for up to 5 weeks.
Oligonucleotide in Situ Hybridization
An oligonucleotide of 45 bases in length for neuron-specific enolase was designed, synthesized, and purified by high-performance liquid chromatography. A total of 500 ng of neuron-specific enolase oligonucleotide was terminally labeled with 50 μCi [33P]dATP by means of terminal deoxynucleotidyl transferase and purified on a Sephadex (Sigma Chemical, St. Louis) G-50 column. Two slides per subject were used for the neuron-specific enolase probe, and in situ hybridization was performed, as we have previously described (46).
Receptor Autoradiography
The pharmacological regulation of the glutamate receptors depends on the unique combination of binding sites on the assembled receptor (42). There is a site on the NMDA receptor for the binding of glutamate, and competitive antagonists of the receptor probably compete with glutamate at this site. A separate glycine binding site must also be occupied before glutamate can activate the ion channel. In addition, there is a site within the ion channel itself that is associated with the binding of noncompetitive antagonists of the NMDA receptor, such as PCP. There is a polyamine modulatory site that is antagonized by the binding of ifenprodil, either directly or through another allosteric site. Assembled AMPA receptors also contain several binding sites: one for glutamate, another at which competitive antagonists such as 6-cyano-7-nitroquinexaline-2,3 dione act (through an allosteric mechanism that affects glutamate binding), and yet another where desensitization modulators exert their influence. Similarly, kainate receptors contain a glutamate binding site; other binding sites have not been as well characterized.
Binding to NMDA, AMPA, and kainate receptors was determined from receptor autoradiography studies, by use of established methods (47–53), which we have described in detail in an earlier report (44). We examined multiple binding sites on the NMDA receptor, including the intrachannel site (visualized with [3H]MK-801), the polyamine site ([3H]ifenprodil), the glutamate site ([3H] CGP39653), and the glycine coagonist site ([3H]MDL105,519). [3H]AMPA and [3H]kainate were used to label those respective receptors. Slides from these studies were exposed to Amersham (Piscataway, N.J.) [3H] Hyperfilm for 1–20 weeks.
Data Analysis
Images were acquired by digitizing in situ hybridization and receptor autoradiography film images with a charged coupled device imaging system. Image analysis was performed by using Image 1.56 (National Institutes of Health, Bethesda, Md.). Thalamic nuclei were identified in each section on the basis of cellular and white-matter patterns, as defined by cresyl violet and gold chloride staining of adjacent sections from each subject (Figure 2) (44, 54). These stained sections were used to identify the thalamus and adjacent structures and to delineate nuclear boundaries within the thalamus. The Y-shaped internal medullary lamina, consisting of afferent and efferent fibers, was used as a major anatomical landmark. It subdivides the thalamus into three gray masses: the anterior, medial, and lateral nuclei (55). Stained slides from each subject were compared to serial thalamic coronal sections from two atlases (55–57). For all subjects, the stained sections studied were at the junction of the anterior and middle third of the thalamus. In each section, the following nuclei were identified for each subject: anterior, dorsomedial, lateral dorsal, central medial, ventral, and reticular. The anterior nucleus lies within the Y bifurcation of the internal medullary lamina and can be subdivided into anteromedial, anteroventral, and anterodorsal nuclei (55). At the level studied, the anteroventral and anteromedial nuclei progressively vanish and are replaced by the lateral dorsal nucleus (55–57). Thus, the “anterior” nucleus in this study mainly represents the anterodorsal nucleus, which was identifiable just beneath the ependymal surface, medial to the dorsomedial nucleus (55–57). The dorsomedial nucleus was well delineated, because it was usually encircled by the internal medullary lamina.
The dorsomedial nucleus consists of the medial magnocellular, dorsolateral parvocellular, and ventral multiform divisions (55). These subdivisions were not clearly distinguishable in the stained sections; thus, all three divisions of the dorsomedial nucleus were pooled for analysis. Beneath the dorsomedial nucleus in the medial plane, the central medial nucleus was identifiable within the internal medullary lamina. Other intralaminar nuclei were visualized in some sections, but only the central medial nucleus was consistently visualized. In this study, the “ventral” nucleus corresponds mainly to the ventral lateral anterior and ventral lateral posterior nuclei (55–58)). These two ventral nuclei were pooled for imaging. The reticular nucleus was clearly visualized as a thin sheet surrounding the dorsal, lateral, and to some extent, the ventral surface of the thalamus (55–57).
For in situ hybridization images, tissue background values were subtracted from gray-scale values and then converted to optical densities. Glutamate receptor subunit mRNA optical densities were then divided by the cyclophilin mRNA optical density for each nucleus so that the dependent variables for analyses were the ratio of glutamate receptor subunit mRNA optical densities over the cyclophilin mRNA optical densities. This was to control for between-subject variability in total mRNA levels. For receptor binding studies, gray-scale values were corrected for nonspecific binding and then converted to optical densities. Values from two sections for each subject from in situ hybridization were averaged and used for data analysis. Statistical analysis was performed for each probe by means of two-way analysis of variance, with nucleus and diagnosis as independent variables. Post hoc analyses were performed by means of the Newman-Keuls test. For all tests, the alpha level was 0.05.
Results
NMDA Receptor Expression
All five subunit mRNAs and all four binding sites were expressed in all nuclei studied (Figure 3 and Figure 4). NMDAR1, NMDAR2A, and NMDAR2B were the most abundant subunit mRNAs, which is consistent with the results from previous studies in the macaque (44, 59). There was no main effect of diagnosis for NMDAR1 mRNA levels, but there was a significant diagnosis-by-nucleus interaction (F=2.42, df=5, 90, p<0.05) (Figure 5). Post hoc analysis revealed significantly lower levels of NMDAR1 mRNA in the subjects with schizophrenia than in comparison subjects in both the dorsomedial and central medial nuclei (p<0.05, Newman-Keuls test). There was also no main effect of diagnosis for NMDAR2B mRNA, but there was a significant diagnosis-by-nucleus interaction (F=4.55, df=5, 90, p<0.001) (Figure 5). Post hoc analysis revealed a significantly lower level of NMDAR2B in the central medial nucleus in the subjects with schizophrenia than in comparison subjects (p<0.01, Newman-Keuls test). There was a significant main effect for diagnosis (F=5.54, df=1, 18, p<0.05) and a significant diagnosis-by-nucleus interaction (F=5.16, df=5, 90, p<0.0005) for NMDAR2C (Figure 5). Post hoc analysis demonstrated significantly lower levels of NMDAR2C mRNA in the anterior (p<0.0005, Newman-Keuls test), dorsomedial (p<0.005, Newman-Keuls test), lateral dorsal (p<0.0005, Newman-Keuls test), and central medial (p<0.0005, Newman-Keuls test) nuclei in the subjects with schizophrenia than in comparison subjects. There were no significant differences in NMDAR2A or NMDAR2D mRNA expression (Figure 5).
There was no main effect of diagnosis for [3H]ifenprodil binding levels, but there was a significant diagnosis-by-nucleus interaction (F=3.33, df=5, 90, p<0.01) (Figure 6). Post hoc analysis demonstrated significantly lower levels of [3H]ifenprodil in anterior (p<0.01, Newman-Keuls test), dorsomedial (p<0.05, Newman-Keuls test), and central medial (p<0.005, Newman-Keuls test) nuclei in the patients than in the comparison subjects. There was a significant main effect of diagnosis for [3H]MDL105,519 binding (F=4.26, df=1, 18, p<0.05), and post hoc analysis demonstrated significantly lower levels of [3H]MDL105,519 binding in the dorsomedial nucleus in the patients than in the comparison subjects (p<0.005, Newman-Keuls test) (Figure 6). There was no significant main effect or diagnosis-by-nucleus interaction for either [3H]MK-801 or [3H]CGP39653 binding levels (Figure 6).
AMPA Receptor Expression
All four AMPA receptor subunit mRNAs and the [3H]AMPA binding site were expressed in all nuclei studied, and at lower levels than NMDA receptor subunit mRNAs and binding sites (Figure 7), which is consistent with the results from previous primate studies (44, 59). There was no main effect of diagnosis for gluR1 mRNA levels, but there was a significant diagnosis-by-nucleus interaction for this subunit mRNA (F=2.46, df=5, 90, p<0.05) (Figure 8). Post hoc analysis demonstrated significantly lower levels of gluR1 mRNA in the dorsomedial (p<0.01, Newman-Keuls test) and central medial (p<0.05, Newman-Keuls test) nuclei in the patients than in the comparison subjects. There was also no main effect of diagnosis for gluR3 mRNA, but there was a significant diagnosis-by-nucleus interaction (F=3.37, df=5, 90, p<0.01) (Figure 8). Post hoc analysis revealed a significantly lower level of gluR3 in the central medial nucleus in the patients than in the comparison subjects (p<0.05, Newman-Keuls test). There were no significant differences in gluR2 mRNA, gluR4 mRNA, or [3H]AMPA binding site expression (Figure 8).
Kainate Receptor Expression
All five subunit mRNAs and the [3H]kainate binding site were expressed in all nuclei studied (Figure 9). This contrasts with results obtained from nonhuman primates, in which gluR6 mRNA is the predominant kainate receptor subunit in the thalamus (44, 59). There was no main effect of diagnosis for KA2 mRNA levels, but there was a significant diagnosis-by-nucleus interaction for this transcript (F=3.46, df=5, 90, p<0.01) (Figure 10). Post hoc analysis demonstrated significantly lower levels of KA2 mRNA in the anterior (p<0.005, Newman-Keuls test), dorsomedial (p<0.0005, Newman-Keuls test), lateral dorsal (p<0.005, Newman-Keuls test), central medial (p<0.0005, Newman-Keuls test), and ventral (p<0.05, Newman-Keuls test) nuclei in the patients than in the comparison subjects. There were no significant differences in gluR5, gluR6, gluR7, or KA1 mRNA expression or in [3H]kainate binding levels (Figure 10).
Neuron-Specific Enolase
There was neither a significant main effect nor a significant diagnosis-by-nucleus interaction in the expression of this neuronal marker (46).
Discussion
In the present study, significantly lower levels of thalamic glutamate receptor expression were detected in the patients with schizophrenia than in the comparison subjects, primarily involving the NMDA receptor and restricted to limbic nuclei. To our knowledge, this is the first demonstration of different ionotropic glutamate receptor expression found in the thalamus in schizophrenia. The differences in NMDA receptor expression are limited to three of the five subunits and two of the four measured binding sites, while abnormalities of AMPA and kainate receptor expression are limited to certain subunit mRNA levels and do not involve differences in final receptor binding site expression. Therefore, there are subunit and binding site-specific abnormalities in ionotropic glutamate receptor expression in the limbic thalamic nuclei in schizophrenia.
The observed differences in NMDA receptor subunit mRNA levels may reflect differences in NMDA receptor composition in the thalamus. NMDA receptor subunit composition confers unique pharmacological characteristics to assembled receptors. NMDAR1 homomers form nonfunctional receptors that bind only glycine (60, 61), and NMDAR2 subunits must coassemble with NMDAR1 for functioning ligand-gated ion channels to result (62, 63). The expression of certain binding sites is associated with specific NMDAR2 subunits, particularly the NMDAR2A subunit with competitive antagonists of the glutamate site and NMDAR2B with the polyamine site (63–65). NMDA receptors containing NMDAR2A or NMDAR2B subunits bind MK-801 more avidly than those containing NMDAR2C or NMDAR2D subunits (63). In vitro studies have suggested that MDL105,519 labels NMDAR1-containing NMDA receptors (53), while ifenprodil labels NMDAR2B-containing NMDA receptors (63, 66). In some thalamic nuclei in the current study, there was concordance between the low transcript and binding site levels found in schizophrenia. Both the dorsomedial nucleus (NMDAR1/[3H]MDL105,519) and the central medial nucleus (NMDAR2B/[3H]ifenprodil) demonstrate this concordance, which is consistent with a postsynaptic localization of these receptors. These results also show that differences in transcription can vary the pharmacological phenotype of NMDA receptor populations.
Our current data suggest that there is lower glycine binding site expression in some thalamic nuclei in schizophrenia patients than in comparison subjects. Classic pharmacology suggests that high thalamic glycine levels might lead to a down-regulation of this site; there are no data that directly address this possibility. Alternatively, this down-regulation may be a pathological response to normal or low glycine levels, leading to hypoactivity of thalamic NMDA receptors. Perhaps the thalamus is one of the anatomical targets for glycine agonist or partial agonist therapy, which may be compensating for low levels of binding sites by maximizing available glycine sites.
Polyamines also facilitate NMDA receptor-mediated transmission at physiological concentrations. Ifenprodil appears to label a site associated with the polyamine site (66–69), so the present data suggest that there is a shift away from polyamine-site-expressing NMDA receptors in the thalamus. As with the glycine site, NMDA receptor neurotransmission may be mitigated in the thalamus in schizophrenia because polyamine facilitation of NMDA receptor currents cannot be fully exploited. Perhaps ligands targeting the polyamine site may also prove therapeutic for schizophrenia.
Both AMPA and kainate receptors may facilitate NMDA receptor-mediated neurotransmission; the NMDA hypoactivity postulated in schizophrenia may be associated with differences in AMPA or kainate receptor expression, rather than with a primary problem with NMDA receptor expression. The low levels of gluR1 and gluR3 found, without a difference in [3H]AMPA binding site levels, suggests that there may be a relatively higher level of gluR2 and gluR4 subunits in assembled AMPA receptors in the thalamus in schizophrenia. Studies have shown that gluR2-containing AMPA receptors have low calcium conductance (16, 70). Therefore, a lower AMPA receptor-mediated activity in schizophrenia patients than in comparison subjects may lead to a lower facilitation of NMDA receptor activity, which is consistent with our hypothesis. Likewise, the lower amount of KA2 subunits seen in our schizophrenia patients than in the comparison subjects may affect kainate receptor-mediated activity. KA2 subunits do not form homomers, but their coassembly with gluR5 or gluR6 subunits leads to kainate receptors with higher conductances (71–73). Consequently, a lower level of KA2 expression may result in a lower facilitation of NMDA receptor activity by kainate receptors. Lower heteroreceptor facilitation of NMDA receptor activity in schizophrenia patients appears to be limited to ionotropic glutamate receptors, since we did not detect any differences in thalamic metabotropic glutamate receptor expression in a separate study of these same subjects (46). Taken together, these data suggest that subtle differences in AMPA and kainate receptor subunit composition may result in lower heteroreceptor facilitation of NMDA receptor activity in the limbic thalamus in schizophrenia patients than in comparison subjects.
Previous studies have demonstrated a lower neuronal number in some thalamic nuclei in schizophrenia patients than in comparison subjects, which we did not detect using the neuronal marker neuron-specific enolase (7, 8, 46, 74). We were only able to examine thalamic nuclei at a single cross-sectional level and did not have the entire extent of the nucleus on which to perform stereology or systemic examination of neuron-specific enolase expression. However, the previously demonstrated lower amounts of both neuron number and total thalamic volume in schizophrenia patients than in comparison subjects may not result in overall differences in cell density, which is consistent with our neuron-specific enolase data. Furthermore, we might expect parallel lower levels in all glutamate receptor subunit mRNAs in schizophrenia patients than in comparison subjects if our current results were just a reflection of lower cell numbers. Therefore, we doubt that putative cellular abnormalities in the thalamus in schizophrenia are the explanation for our findings, although our data could be consistent with a selective loss of a subpopulation of thalamic neurons that selectively express NMDAR1 and NMDAR2C subunits.
Lower levels of expression of ionotropic glutamate receptor subunit mRNAs and NMDA receptor binding sites in schizophrenia patients than in comparison subjects appear to be restricted to glutamatergic relay nuclei, which are reciprocally connected with structures that have been implicated in schizophrenia. The centromedial nucleus, which is a major thalamic relay between prefrontal, cingulate, and other limbic cortical areas (55, 75) and the nucleus accumbens (76), has significantly lower levels of expression of NMDAR1, NMDAR2B, NMDAR2C, gluR1, gluR3, and KA2 mRNA and [3H]ifenprodil binding in schizophrenia patients than in comparison subjects. The dorsomedial nucleus, which projects primarily to the prefrontal cortex (77, 78) and receives input from the amygdala, cortical areas, and the midbrain (79–81), shows lower levels of expression of NMDAR1, NMDAR2C, gluR1, and KA2 mRNAs and [3H]ifenprodil and [3H]MDL105,519 binding sites in schizophrenia patients than in comparison subjects. The anterior nucleus, which projects to the cingulate gyrus (82, 83) and receives input primarily from the subiculum (82, 83), expresses lower levels of NMDAR2C mRNA and [3H]ifenprodil binding in schizophrenia, and the lateral dorsal nucleus, which receives input from the hippocampus and projects to the cingulate gyrus (82, 83), shows lower NMDAR2C and KA2 mRNA expression. Our data reveal low levels of KA2 mRNA only in the ventral nuclei of schizophrenia patients, which project to somatosensory, motor, and premotor cortical areas (55, 84, 85); these regions are probably not implicated in the pathophysiology of schizophrenia. Similarly, our data reveal no lower levels of binding or transcript expression in the reticular nuclei of schizophrenia patients, which utilize GABAergic projections to other thalamic nuclei (55, 86). There appears to be a continuum of abnormal glutamate receptor expression, with regions projecting to the prefrontal cortex having the greatest degree of abnormality, to nonlimbic thalamic areas having few, if any, abnormalities. These findings suggest that low glutamate receptor expression occurs in the thalamic areas that are components of complex cortical and subcortical circuitry associated with the pathophysiology of schizophrenia.
The hypoactivity of the thalamus seen in imaging studies of schizophrenia may be associated with concomitant low level of neurons. However, our present data suggest that the hypoactivity may also be related to a lower level of NMDA receptor-mediated activity. NMDA receptors are the predominant transducer of glutamatergic neurotransmission in the thalamus (17), so an illness-associated low level of NMDA receptor activity may manifest as low metabolic activity in the thalamus as well as in its efferent targets.
This study has a number of limitations that should be considered when interpreting these data. As in most studies of schizophrenia that rely on postmortem tissue, there is the potential confounding variable of antipsychotic exposure. Although it is clear that antipsychotic exposure may alter thalamic metabolism and immediate early gene expression (87–90), antipsychotics have not been found, at least in one study (91), to regulate NMDA receptor expression. Nonetheless, some of these results could be due to the effects of chronic antipsychotic treatment. Similarly, these results could be in part attributable to the effects of chronic institutionalization, as this is an older inpatient population; the comparison group was from neighboring nursing homes, and this may at least in part control for some aspects of residential care. It should be appreciated that these data are from an older cohort of subjects, and although the resulting data are perhaps a fair reflection of thalamic neurochemical anatomy in schizophrenia in later life, they may not generalize to younger patients. Accordingly, although these findings are intriguing, whether they are primarily due to schizophrenia or are a secondary condition associated with having this chronic illness for many decades cannot be answered by these results.
Our results demonstrate that glutamate receptor expression in the thalamus of schizophrenia patients is different from that found in the thalamus of comparison subjects at both transcriptional and posttranscriptional levels, but the differences are most prominent in nuclei with reciprocal projections to limbic regions. These results also suggest that differences in NMDA receptor subunit mRNA levels affect the expression of polyamine and glycine binding sites in the thalamus of schizophrenia patients, highlighting the importance of examining receptor expression at multiple levels of gene expression. Combination therapy with positive modulators of both the glycine and polyamine sites of NMDA receptors may prove to be an efficacious treatment strategy for schizophrenia.
 |
Presented at the biennial meeting of the International Congress on Schizophrenia Research, Santa Fe, N.M., April 17–21, 1999. Received Aug. 19, 1999; revision received June 5, 2000; accepted June 7, 2000. From the Department of Psychiatry and the Mental Health Research Institute, University of Michigan; and the Department of Psychiatry, Mount Sinai School of Medicine, New York. Address reprint requests to Dr. Meador-Woodruff, Mental Health Research Institute and Department of Psychiatry, University of Michigan, 205 Zina Pitcher Place, Ann Arbor, MI 48109-0720; [email protected] (e-mail). Supported by NIHM grant MH-53327 and a National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award (to Dr. Meador-Woodruff); an NARSAD Young Investigator Award and an American Psychiatric Association/Lilly Psychiatric Fellowship (to Dr. Healy); a VA Merit Review Award (to Dr. Haroutunian); and grants from NIHM (MH-45212) and the National Institute on Aging (AG-5138 and AG-2219) (to Dr. Davis).
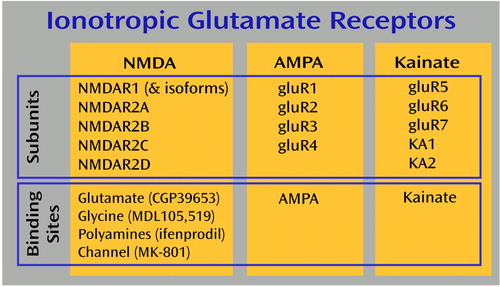
Figure 1. Summary of the Relationships of Ionotropic Glutamate Receptor Subunits and Binding Sites in the Braina
aMPA and kainate binding sites are labeled with [ 3H]AMPA and [3H]kainate, respectively. The binding sites of the NMDA receptors are labeled with tritiated forms of the compounds shown in parentheses.
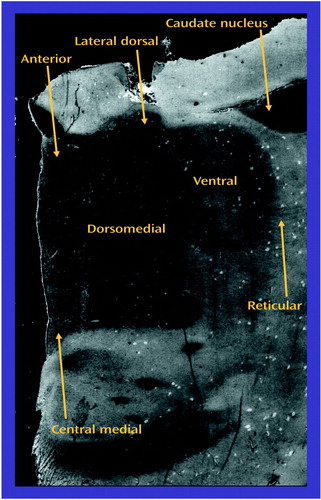
Figure 2. Cresyl-Violet-Stained Section of a Typical Thalamic Sectiona
aThe tail of the caudate nucleus of the striatal complex is seen at this level.
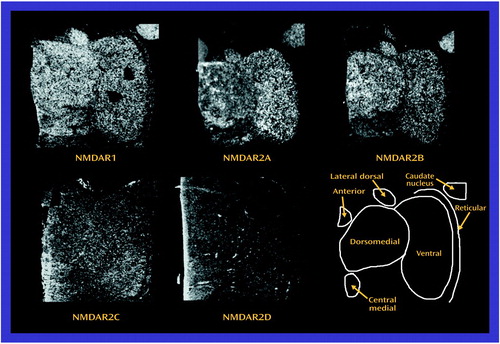
Figure 3. Distribution of N-Methyl-d-Aspartic Acid (NMDA) Receptor Subunit mRNA in Thalamic Nucleia
aAll five subunit mRNAs were detectable, and NMDAR1, NMDAR2A, and NMDAR2B were the most abundant.
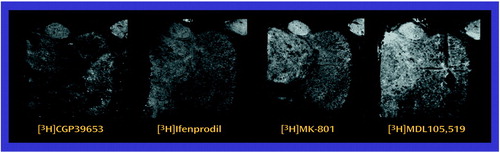
Figure 4. Distribution of N-Methyl-d-Aspartic Acid (NMDA) Receptor Binding Sites in Thalamic Nuclei
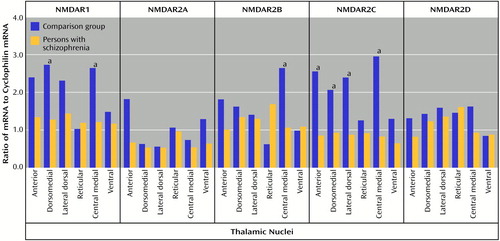
Figure 5. N-Methyl-d-Aspartic Acid (NMDA) Receptor Subunit mRNA Levels in the Thalamic Nuclei of Schizophrenia Patients and Comparison Subjects
aSignificant difference between the groups (analysis of variance and post hoc contrasts by means of the Newman-Keuls test).
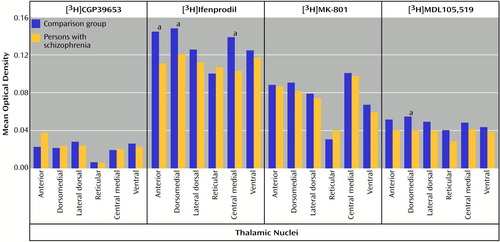
Figure 6. N-Methyl-d-Aspartic Acid (NMDA) Receptor Binding Levels in the Thalamic Nuclei of Schizophrenia Patients and Comparison Subjects
aSignificant difference between the groups (analysis of variance and post hoc contrasts by means of the Newman-Keuls test).
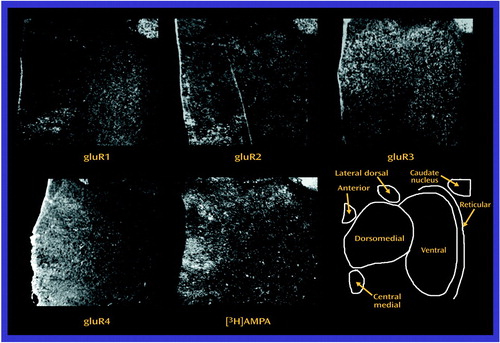
Figure 7. Distribution of AMPA Receptor Subunit mRNA and the [3H]AMPA Binding Site in Thalamic Nucleia
aAll four subunit mRNAs and the [3H]AMPA binding site were detectable but all at very low levels of expression relative to the NMDA receptor subunit mRNAs and binding sites.
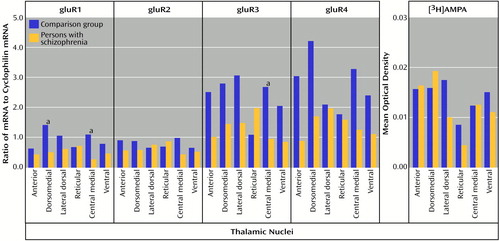
Figure 8. AMPA Receptor Subunit mRNA and [3H]AMPA Binding Levels in the Thalamic Nuclei of Schizophrenia Patients and Comparison Subjects
aSignificant difference between the groups (analysis of variance and post hoc contrasts by means of the Newman-Keuls test).
Figure 9. Distribution of Kainate Receptor Subunit mRNA and the [ 3H]Kainate Binding Site in Thalamic Nuclei
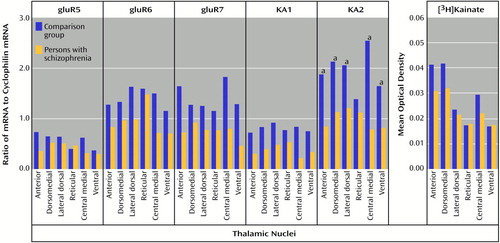
Figure 10. Kainate Receptor Subunit mRNA and [3H]Kainate Binding Levels in the Thalamic Nuclei of Schizophrenia Patients and Comparison Subjects
aSignificant difference between the groups (analysis of variance and post hoc contrasts by means of the Newman-Keuls test).
1. Steriade M, Jones EG, McCormick DA: The Thalamus. Oxford, UK, Elsevier, 1997Google Scholar
2. Andreasen NC, Arndt S, Swayze V II, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WTC: Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994; 266:294–298Crossref, Medline, Google Scholar
3. Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Boles Ponto LL, Watkins GL, Hichwa RD: Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 1996; 93:9985–9990Google Scholar
4. Andreasen NC, Paradiso S, O’Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203–218Google Scholar
5. Buchsbaum MS, Someya T, Ying Teng CY, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE Jr: PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 1996; 153:191–199Link, Google Scholar
6. Buchsbaum MS, Hazlett EA: Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr Bull 1998; 24:343–364Crossref, Medline, Google Scholar
7. Pakkenberg B: Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 1990; 47:1023–1028Google Scholar
8. Pakkenberg B: The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr Res 1992; 7:95–100Crossref, Medline, Google Scholar
9. Vita A, Bressi S, Perani D, Invernizzi G, Giobbio GM, Dieci M, Garbarini M, Del Sole A, Fazio F: High-resolution SPECT study of regional cerebral blood flow in drug-free and drug-naive schizophrenic patients. Am J Psychiatry 1995; 152:876–882Link, Google Scholar
10. Arciniegas D, Rojas DC, Teale P, Sheeder J, Sandberg E, Reite M: The thalamus and the schizophrenia phenotype: failure to replicate reduced volume. Biol Psychiatry 1999; 45:1329–1335Google Scholar
11. Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG, Fischer I, Kikinis R, Donnino R, Jolesz FA, McCarley RW: Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry 1998; 43:649–659Crossref, Medline, Google Scholar
12. Staal WF, Hulshoff Pol HE, Schnack H, van der Schot AC, Kahn RS: Partial volume decrease of the thalamus in relatives of patients with schizophrenia. Am J Psychiatry 1998; 155:1784–1786Google Scholar
13. Hazlett EA, Buchsbaum MS, Byne W, Wei T-C, Speigel-Cohen J, Geneve C, Kinderlehrer R, Havnedar MM, Shihbuddin L, Siever LJ: Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry 1999; 156:1190–1199Google Scholar
14. Broman J: Neurotransmitters in subcortical somatosensory pathways. Anat Embryol (Berl) 1994; 189:181–214Crossref, Medline, Google Scholar
15. Kharazia VN, Weinberg RJ: Glutamate in thalamic fibers terminating in layer IV of primary sensory cortex. J Neurosci 1994; 14:6021–6032Google Scholar
16. Hollmann M, Heinemann S: Cloned glutamate receptors. Annu Rev Neurosci 1994; 17:31–108Crossref, Medline, Google Scholar
17. Salt TE, Eaton SA: Functions of ionotropic and metabotropic glutamate receptors in sensory transmission in the mammalian thalamus. Prog Neurobiol 1996; 48:55–72Crossref, Medline, Google Scholar
18. Itil T, Keskiner A, Kiremitci N, Holden JMC: Effect of phencyclidine in chronic schizophrenia. Can J Psychiatry 1967; 12:209–212Google Scholar
19. Javitt DC, Zukin SR: Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991; 148:1301–1308Google Scholar
20. Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS: Subanesthetic effects of the noncompetitive NMDA antagonist ketamine in humans: psychotomimetic perceptual cognitive and neuroendocrine responses. Arch Gen Psychiatry 1994; 51:199–214Crossref, Medline, Google Scholar
21. Lahti AC, Holcomb HH, Medoff DR, Tamminga CA: Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport 1995; 6:869–872Crossref, Medline, Google Scholar
22. Luby ED, Gottlieb JS, Cohen BD, Rosenbaum G, Domino EF: Model psychoses and schizophrenia. Am J Psychiatry 1962; 119:61–67Link, Google Scholar
23. Goff DC, Tsai G, Manoach DS, Coyle JT: Dose-finding trial of d-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry 1995; 152:1213–1215Google Scholar
24. Goff DC, Tsai G, Manoach DS, Coyle JT: A placebo-controlled trial of d-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry 1999; 56:21–27Crossref, Medline, Google Scholar
25. Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D: Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment resistant schizophrenia. Br J Psychiatry 1996; 169:610–617Crossref, Medline, Google Scholar
26. Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M: Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry 1999; 56:29–36Crossref, Medline, Google Scholar
27. Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Lindenmayer J-P: Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry 1994; 151:1234–1236Google Scholar
28. Rosse RB, Fay-McCarthy M, Kendrick K, Davis RE, Deutsch SI: d-Cycloserine adjuvant therapy to molindone in the treatment of schizophrenia. Clin Neuropharmacol 1996; 19:444–450Crossref, Medline, Google Scholar
29. Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM: Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 1996; 379:78–81Crossref, Medline, Google Scholar
30. Clarke VRJ, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D: A hippocampal gluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 1997; 389:599–603Crossref, Medline, Google Scholar
31. Collins GGS, Anson J, Surtees L: Presynaptic kainate and N-methyl-d-aspartate receptors regulate excitatory amino acid release in the olfactory cortex. Brain Res 1983; 265:157–159Crossref, Medline, Google Scholar
32. Cunha RA, Constantino MD, Ribeiro JA: Inhibition of [3H] gamma aminobutyric acid release by kainate receptor activation in rat hippocampal synaptosomes. Eur J Pharmacol 1997; 323:167–172Crossref, Medline, Google Scholar
33. Ferkany JW, Zaczek R, Coyle JT: Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors. Nature 1982; 298:757–759Crossref, Medline, Google Scholar
34. Frerking M, Malenka RC, Nicoll RA: Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci 1998; 1:479–486Crossref, Medline, Google Scholar
35. Lodge D, Bleakman D: A hippocampal gluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 1997; 389:599–603Crossref, Medline, Google Scholar
36. Rodriguez-Moreno A, Herreras O, Lerma J: Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 1997; 19:893–901Crossref, Medline, Google Scholar
37. He Y, Janssen WGM, Morrison JH: Synaptic coexistence of AMPA and NMDA receptors in the rat hippocampus: a postembedding immunogold study. J Neurosci Res 1998; 54:444–449Crossref, Medline, Google Scholar
38. Alagarsamy S, Marino MJ, Rouse ST, Gereau RW, Heinemann SF, Conn PJ: Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci 1999; 2:234–240Crossref, Medline, Google Scholar
39. Kishi A, Ohno M, Watanabe S: Spermidine, a polyamine site agonist, attenuates working memory deficits caused by blockade of hippocampal muscarinic receptors and mGluRs in rats. Brain Res 1998; 793:311–314Crossref, Medline, Google Scholar
40. Moghaddam B, Adams BW: Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 1998; 281:1349–1352Google Scholar
41. Durand GM, Bennett MVL, Zukin RS: Splice variants of the N-methyl-d-aspartate receptor NMDAR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci USA 1993; 90:6731–6735Google Scholar
42. Wheal HV, Thomson AM: Excitatory Amino Acids and Synaptic Transmission. London, Academic Press, 1995Google Scholar
43. Meador-Woodruff JH, King RE, Damask SP, Bovenkerk KA: Differential regulation of hippocampal AMPA and kainate receptor subunit expression by haloperidol and clozapine. Mol Psychiatry 1997; 1:41–53Google Scholar
44. Ibrahim HM, Healy DJ, Hogg AJ, Meador-Woodruff JH: Nucleus-specific expression of ionotropic glutamate receptor subunit mRNAs and binding sites in primate thalamus. Brain Res Mol Brain Res 2000; 79:1–17Crossref, Medline, Google Scholar
45. Danielson PE, Forss-Petter S, Brow MA, Calavetta L, Douglass J, Milner RJ, Sutcliffe JG: p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA 1988; 7:261–267Crossref, Medline, Google Scholar
46. Richardson-Burns SM, Haroutunian V, Davis KL, Watson SJ, Meador-Woodruff JH: Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus. Biol Psychiatry 2000; 47:22–28Crossref, Medline, Google Scholar
47. Huettner JE, Bean BP: Block of N-methyl-d-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci USA 1987; 85:1307–1311Google Scholar
48. Ransom RW, Stec NL: Cooperative modulation of [3H]MK-801 binding to the N-methyl-d-aspartate receptor-ion channel complex by l-glutamate, glycine, and polyamines. J Neurochem 1988; 51:830–836Crossref, Medline, Google Scholar
49. Hashimoto K, Mantione CR, Spada MR, Neumeyer JL, London ED: Further characterization of [3H]ifenprodil binding in rat brain. Eur J Pharmacol 1994; 266:67–77Crossref, Medline, Google Scholar
50. Sills MA, Fagg G, Pozza M, Angst C, Brundish DE, Hurt SD, Wilusz EJ, Williams M: [3H]CGP39653: a new N-methyl-d-aspartate antagonist radioligand with low nanomolar affinity in rat brain. Eur J Pharmacol 1991; 192:19–24Crossref, Medline, Google Scholar
51. White BH, Vogel MW: CGP 39653 binding in the chick CNS after NMDA receptor antagonist treatment. J Neural Transm 1996; 103:1247–1253Google Scholar
52. Baron BM, Siegel BW, Harrison BL, Gross RS, Hawes C, Towers P: [3H]MDL105,519, a high-affinity radioligand for the N-methyl-d-aspartate receptor-associated glycine recognition site. J Pharmacol Exp Ther 1996; 279:62–68Medline, Google Scholar
53. Siegel BW, Sreekrishna K, Baron BM: Binding of the radiolabelled glycine site antagonist [3H]MDL105,519 to homomeric NMDA-R1a receptors. Eur J Pharmacol 1996; 312:357–365Crossref, Medline, Google Scholar
54. Schmued LC: A rapid, sensitive histochemical stain for myelin in frozen brain sections. J Histochem Cytochem 1990; 38:717–720Crossref, Medline, Google Scholar
55. Hirai T, Jones EG: A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res 1989; 14:1–34Crossref, Google Scholar
56. Mai JK, Assheuer J, Paxinos G: Atlas of the Human Brain. San Diego, Calif, Academic Press, 1997Google Scholar
57. Swanson LW, Cowan WM: An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 1977; 172:49–84Crossref, Medline, Google Scholar
58. Donavan MK, Wyss JM: Evidence for some collateralization between cortical and diencephalic efferent axons of the rat subicular cortex. Brain Res 1983; 259:181–192Crossref, Medline, Google Scholar
59. Jones EG, Tighilet B, Tran B-V, Huntsman MM: Nucleus- and cell-specific expression of NMDA and non-NMDA receptor subunits in monkey thalamus. J Comp Neurol 1998; 397:371–393Crossref, Medline, Google Scholar
60. Boeckman FA, Aizenman E: Stable transfection of the NMDAR1 subunit in Chinese hamster ovary cells fails to produce a functional N-methyl-d-aspartate receptor. Neurosci Lett 1994; 173:189–192Crossref, Medline, Google Scholar
61. Grimwood S, LeBourdelles B, Whiting PJ: Recombinant human NMDA homomeric NMDAR1 receptors expressed in mammalian cells form a high-affinity glycine antagonist binding site. J Neurochem 1995; 64:525–530Crossref, Medline, Google Scholar
62. Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH: Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 1992; 256:1217–1220Google Scholar
63. Lynch DR, Anegawa NJ, Verdoorn T, Pritchett DB: N-Methyl-d-aspartate receptors: different subunit requirements for binding of glutamate antagonists, glycine antagonists, and channel-blocking agents. Mol Pharmacol 1994; 45:540–545Medline, Google Scholar
64. Gallagher MJ, Huang H, Pritchett DB, Lynch DR: Interactions between ifenprodil and the NMDAR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 1996; 271:9603–9611Google Scholar
65. Varney MA, Jachec C, Deal C, Hess SD, Daggett LP, Skvoretz R, Urcan M, Morrison JH, Moran T, Johnson EC, Velicelebi G: Stable expression and characterization of recombinant human heteromeric N-methyl-d-aspartate receptor subtypes NMDAR1A/2A and NMDAR1A/2B in mammalian cells. J Pharmacol Exp Ther 1996; 279:367–378Medline, Google Scholar
66. Williams K: Interactions of polyamines with ion channels. Biochem J 1997; 325:289–297Crossref, Medline, Google Scholar
67. Kew JN, Trube G, Kemp JA: A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J Physiol (Lond) 1996; 497:761–772Crossref, Google Scholar
68. Legendre P, Westbrook GL: Ifenprodil blocks N-methyl-d-aspartate receptors by a two component mechanism. Mol Pharmacol 1991; 40:289–298Medline, Google Scholar
69. Reynolds IJ, Miller RJ: Ifenprodil is a novel type of N-methyl-d-aspartate receptor antagonist: interaction with polyamines. Mol Pharmacol 1989; 36:758–765Medline, Google Scholar
70. Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H: Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 1994; 12:1281–1289Google Scholar
71. Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH: The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron 1992; 8:775–785Crossref, Medline, Google Scholar
72. Sommer B, Burnashev N, Verdoorn TA, Keinanen K, Sakmann B, Seeburg PH: A glutamate receptor channel with high affinity for domoate and kainate. EMBO J 1992; 11:1651–1656Google Scholar
73. Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG: Effect of RNA editing and subunit co-assembly single-channel properties of recombinant kainate receptors. J Physiol (Lond) 1996; 492:129–142Crossref, Google Scholar
74. Danos P, Baumann B, Bernstein H-G, Franz M, Stauch R, Northoff G, Krell D, Falkai P, Bogerts B: Schizophrenia and anteroventral thalamic nucleus: selective decrease of parvalbumin-immunoreactive thalamocortical projection neurons. Psychiatry Res Neuroimaging 1998; 82:1–10Crossref, Medline, Google Scholar
75. Royce GJ, Bromley S, Gracco C, Beckstead RM: Thalamocortical connections of the rostral intralaminar nuclei: an autoradiographic analysis in the cat. J Comp Neurol 1989; 288:555–582Crossref, Medline, Google Scholar
76. Gimenez-Amaya JM, McFarland MR, De Las Heras S, Haber SN: Organization of thalamic projections to the ventral striatum in the primate. J Comp Neurol 1995; 354:127–149Crossref, Medline, Google Scholar
77. Ray JP, Price JL: The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 1993; 337:1–31Crossref, Medline, Google Scholar
78. Goldman-Rakic PS, Porrino LJ: The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol 1985; 242:535–560Crossref, Medline, Google Scholar
79. Kuroda M, Price JL: Synaptic organization of projections from basal forebrain structures to the mediodorsal thalamic nucleus of the rat. J Comp Neurol 1991; 303:513–533Crossref, Medline, Google Scholar
80. Kuroda M, Muradami K, Kishi K, Price JL: Distribution of the piriform cortical terminals to cells in the central segment of the mediodorsal thalamic nucleus of the rat. Brain Res 1992; 595:159–163Crossref, Medline, Google Scholar
81. Ray JP, Russhen FT, Fuller TA, Price JL: Sources of presumptive glutamatergic/aspartatergic afferents to the mediodorsal nucleus of the thalamus in the rat. J Comp Neurol 1992; 320:435–456Crossref, Medline, Google Scholar
82. Shibata H: Efferent projection from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurol 1993; 330:533–542Crossref, Medline, Google Scholar
83. Van Groen T, Wyss JM: Projections from the laterodorsal nucleus of the thalamus to the limbic and visual cortices in the rat. J Comp Neurol 1992; 324:427–448Crossref, Medline, Google Scholar
84. Sakai ST, Inase M, Tanji J: Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. J Comp Neurol 1996; 368:215–228Crossref, Medline, Google Scholar
85. Rouiller EM, Liang F, Bahalian A, Moret V, Wiesendanger M: Cerebellothalamocortical and pallidothalamocortical projections to the primary and supplementary motor cortical areas: a multiple tracing study in macaque monkeys. J Comp Neurol 1994; 345:185–213Crossref, Medline, Google Scholar
86. Cornwall J, Cooper JD, Phillipson OT: Projections to the rostral reticular thalamic nucleus in the rat. Exp Brain Res 1990; 80:157–171Crossref, Medline, Google Scholar
87. Cohen BM, Wan W: The thalamus as a site of action of antipsychotic drugs. Am J Psychiatry 1996; 153:104–106Link, Google Scholar
88. Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA: Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry 1996; 153:41–49Link, Google Scholar
89. Lavin A, Grace AA: Response of the ventral pallidal/mediodorsal thalamic system to antipsychotic drug administration: involvement of the prefrontal cortex. Neuropsychopharmacology 1998; 18:352–363Crossref, Medline, Google Scholar
90. Deutch AY, Ongur D, Duman RS: Antipsychotic drugs induce Fos protein in the thalamic paraventricular nucleus: a novel of antipsychotic drug action. Neuroscience 1995; 66:337–346Crossref, Medline, Google Scholar
91. Ulas J, Nguyen L, Cotman CW: Chronic haloperidol treatment enhances binding to NMDA receptors in rat cortex. Neuroreport 1993; 4:1049–1051Google Scholar



