White Matter Abnormalities in First-Episode, Treatment-Naive Young Adults With Major Depressive Disorder
Abstract
Objective: The aim of this study was to explore the microstructural integrity of whole-brain white matter by diffusion tensor imaging in first-episode, treatment-naive young adults with major depressive disorder. Method: Diffusion tensor imaging scans were obtained from 14 first-episode, treatment-naive young adult patients with major depressive disorder and 14 healthy comparison subjects. A voxel-based method was used to analyze the scans. Results: The patient group exhibited significantly lower fractional anisotropy values than healthy comparison subjects in the white matter of the right middle frontal gyrus, the left lateral occipitotemporal gyrus, and the subgyral and angular gyri of the right parietal lobe. There were no regions of significantly higher fractional anisotropy values in patients compared with healthy comparison subjects. Conclusions: These findings suggest that abnormalities of brain white matter may be present early in the course of major depressive disorder. They also support the idea that white matter lesions may disrupt the neural circuits involved in mood regulation and thus contribute to the neuropathology of major depressive disorder.
Abnormalities in frontal-subcortical circuits may play an important role in the pathophysiology of major depressive disorder (1 – 4) . Magnetic resonance imaging (MRI) studies of depression have demonstrated reduced gray matter volumes in the prefrontal cortex (5 – 7) , including the middle frontal gyrus (8) , anterior cingulate, gyrus rectus, and orbitofrontal cortex (9) . Also, deep white matter hyperintensities (5 , 10 , 11) , especially at the level of the dorsolateral prefrontal cortex, have been reported in elderly depressed patients (12) . Prefrontal abnormalities in both gray and white matter are most strongly associated with cognitive and emotional dysfunction in depression (2 , 13) .
Diffusion tensor imaging, a newly developed MRI technique, can provide information about white matter microstructural integrity in vivo by measuring the magnitude and direction of water diffusion. Recently, diffusion tensor imaging studies using region-of-interest methods in elderly depressed patients found microstructural white matter abnormalities in frontal and temporal lobes (14 – 17) , although in one of these studies (14) the influence of antidepressant medications could not be excluded. Despite growing evidence for prefrontal cortical abnormalities in young as well as in elderly depressed patients (18) , there are no published diffusion tensor imaging studies examining the integrity of whole-brain white matter in first-episode, treatment-naive young adult patients with major depressive disorder. We designed the pilot study reported here to further understand the neuropathology of major depressive disorder by using voxel-based analysis (19–21), a method that can assess comprehensive global brain structure changes without the restrictions imposed by the prior selection of regions of interest and thus potentially identify unsuspected anatomic abnormalities in the brain (22) .
Method
Subjects
Fourteen right-handed outpatients with major depressive disorder (12 of them female) age 20–41 years (mean=28.9, SD=8.0) were recruited at Second Xiangya Hospital of Central South University in Changsha, Hunan, China. They were interviewed with the Structured Clinical Interview for DSM-IV. All patients were experiencing their first episode of major depressive disorder and were treatment naive. The mean age at illness onset was 28.1 years (SD=7.8) and the mean illness duration was 10.3 months (range=3 to 24, SD=8.3). Scores on the Beck Depression Inventory (BDI) at the time of the study ranged from 27 to 48 (mean=37.5, SD=6.9). Fourteen matched right-handed healthy comparison subjects (12 of them female) age 19–41 years (mean=27.1, SD=6.7) were also recruited. The two groups had similar years of education (patient group, mean=11.4 years [SD=3.5]; comparison group, mean=12.0 years [SD=2.8]; p>0.05).
Exclusion criteria for both patients and comparison subjects were any history of loss of consciousness, meeting DSM-IV criteria for substance abuse within the 6 months prior to the scanning, mental retardation, or a current serious medical or neurological illness. An additional exclusion criterion for the patient group was any lifetime psychiatric disorder other than major depressive disorder. Additional exclusion criteria for comparison subjects were any history of psychiatric illness or a family history of major psychiatric or neurological illness in first-degree relatives. All subjects were given information about the procedures and gave written informed consent via forms approved by the Ethics Committee of the Second Xiangya Hospital of Central South University.
Image Acquisition and Processing
Diffusion tensor imaging was performed on a 1.5-T GE scanner (Twinspeed, Milwaukee). A birdcage head coil was used, along with restraining foam pads to minimize head motion. Single-shot echo planar diffusion-weighted imaging with alignment of the anterior commissure-posterior commissure plane was done, using the following parameters: repetition time=12,000 msec; echo time=105 msec; acquisition matrix=128×128; field of view=24×24 cm; number of excitations=5; slice thickness=4 mm, no gap, 30 contiguous axial slices. The diffusion sensitizing gradients were applied along 13 noncollinear directions (b=1000 s/mm 2 ), together with an acquisition without diffusion weighting (b=0).
The diffusion tensor matrix was calculated according to the equation in Basser et al.’s study (23) . Three pairs of eigenvalues (λ 1 , λ 2 , λ 3 ) and eigenvectors can be obtained by diagonalization of the tensor matrix. The principal direction at each point was given by the eigenvector that corresponds to the largest eigenvalue, and a fractional anisotropy (FA) value (24) was calculated according to the following formula:
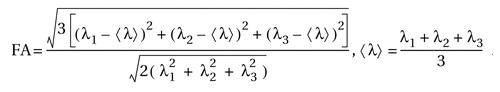
For each subject, statistical parametric mapping (SPM2, Wellcome Department of Cognitive Neurology, London) was first used to normalize the b=0 image to the standard Montreal Neurological Institute (MNI) space by nonlinear registration, and then the transformation matrix was applied to the FA map in order to normalize the map to the standard MNI space. All images were resampled with a final voxel size of 2×2×2 mm 3 , and a 6-mm full-width half-maximum Gaussian kernel was used with the FA map to decrease spatial noise and smooth the data. No correction for eddy current distortions was applied.
Statistical Analysis
Two-sample t tests were performed in a voxel-by-voxel manner. A p value (two-tailed) lower than 0.001 (uncorrected) in voxel difference and a cluster size greater than 50 voxels were considered to be statistically significant. To visualize regions showing significantly different FA values, significant clusters were superimposed onto SPM2’s spatially normalized template brain.
Results
In a voxel-by-voxel contrast, compared with healthy comparison subjects, patients with major depressive disorder showed significantly lower FA values in four brain white matter regions ( Figure 1 ): the right middle frontal gyrus, the left lateral occipitotemporal gyrus, the subgyral white matter of the right parietal lobe (near the sulcus callosomarginalis and partially in the precuneus), and the right angular gyrus. The Talairach coordinates for the peak voxel in the four brain regions, respectively, were x=36, y=49, z=10; x=–42, y= –56, z=–1; x=24, y=–47, z=41; and x=42, y=–65, z=27. All four regions showed large effect sizes (1.918, 1.961, 1.789, and 2.051, respectively), as calculated with the equation d=2t/√(df) (25) . Compared with healthy comparison subjects, patients with major depressive disorder did not show significantly higher FA values in any brain regions. Furthermore, no brain areas with significantly different FA values were found between patients with short (N=8) and long (N=6) illness durations (cutoff at 12 months), or between those with low (N=6) and high (N=8) BDI scores (cutoff at 38).
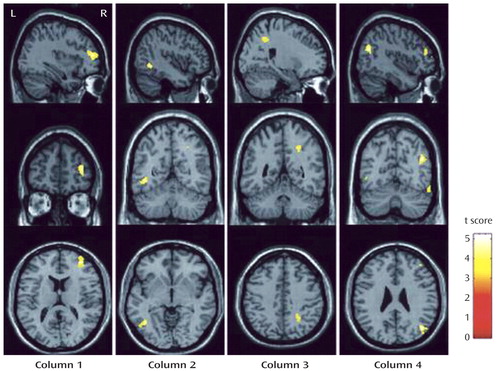
a The brain regions identified were the white matter in the right middle frontal gyrus (column 1) (t=4.89, df=26, p<0.001, cluster size=258), the left lateral occipitotemporal gyrus (column 2) (t=5.00, df=26, p<0.001, cluster size=96), the subgyral white matter of the right parietal lobe (near the sulcus callosomarginalis and partially in the precuneus) (column 3) (t=4.56, df=26, p<0.001, cluster size=76), and the right angular gyrus (column 4) (t=5.23, df=26, p<0.001, cluster size=120).
Discussion
Previous diffusion tensor imaging studies using region-of-interest methods with elderly depressed patients found white matter abnormalities in widespread frontal brain regions (14 – 17) . Additionally, Bell-McGinty et al. (8) reported smaller white matter volumes in the right middle frontal gyrus in geriatric depressed patients than in healthy comparison subjects by voxel-based morphometry. Our study similarly demonstrated white matter abnormalities in the right middle frontal gyrus in young adult depressed patients. The right middle frontal gyrus corresponds anatomically to the right dorsolateral prefrontal cortex, which is involved functionally in cognition and emotional regulation (4) and has been found to show hypometabolism both in depressed subjects before treatment (26 – 28) and in healthy comparison subjects during experimentally induced sadness (28) . Even prefrontal lobe volume loss has been observed in late-onset and early-onset depression (7) . Taken together, these findings suggest that loss of integrity in prefrontal white matter may exist in depression in a variety of age groups.
We observed white matter abnormalities in the left lateral occipitotemporal gyrus, which is the lateral portion of the fusiform gyrus and has extensive afferent and efferent connections with the limbic regions. Our findings are supported by two region-of-interest studies (15 , 16) that reported reduced temporal lobe white matter FA values in depressed patients and by neuroimaging studies that reported hypometabolism (29) and abnormal neural activity (30) in the left fusiform gyrus in depression. We also found abnormalities in the right parietal white matter among depressed subjects, which is congruent with previous studies using positron emission tomography (28 , 31) , although this finding is inconsistent with that reported by Nobuhara et al. (15) . The small sample size in our pilot study might have contributed to such differences. In addition, many other factors, including the mean age of subjects, age at onset of depression, illness duration, depression severity, drug administration, and methods of imaging data processing and analysis, could contribute to the inconsistent findings. Taken together, however, these findings support the theory that white matter lesions disrupt the neural circuits involved in mood regulation, which would contribute to the pathogenesis of major depressive disorder.
To our knowledge, this study is the first to use voxel-based analysis to explore the integrity of whole-brain white matter microstructure in first-episode, treatment-naive young adult patients with major depressive disorder. Its principal limitations are the relatively small sample size, the large range of illness duration (3–24 months), and the risk of a type I error (uncorrected). Significant clusters in our pilot study would not survive after false discovery rate correction. Furthermore, compared with the region-of-interest method, the voxel-based method cannot focus on specific brain regions and calculate concrete means and standard deviations of FA values in each cluster. Despite the limitations, using the voxel-based method with a relatively strict restriction of p<0.001 and a cluster size >50 voxels, we present evidence for possible loss of integrity of white matter fiber tracts in frontal, temporal, and parietal lobes in first-episode, treatment-naive young adult patients with major depressive disorder, which suggests that white matter pathology may occur early in the course of illness. Future studies can use diffusion tensor imaging to explore whether such abnormalities are progressive over the course of major depressive disorder as well as the relationship between these abnormalities and depression severity, response to treatment, and risk of relapse.
1. Drevets WC: Neuroimaging studies of mood disorders. Biol Psychiatry 2000; 48:813–829Google Scholar
2. Tekin S, Cummings JL: Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 2002; 53:647–654Google Scholar
3. Sheline YI: Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 2003; 54:338–352Google Scholar
4. Phillips ML, Drevets WC, Rauch SL, Lane R: Neurobiology of emotion perception, II: implications for major psychiatric disorders. Biol Psychiatry 2003; 54:515–528Google Scholar
5. Coffey CE, Wilkinson WE, Weiner RD, Parashos IA, Djang WT, Webb MC, Figiel GS, Spritzer CE: Quantitative cerebral anatomy in depression: a controlled magnetic resonance imaging study. Arch Gen Psychiatry 1993; 50:7–16Google Scholar
6. Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME: Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824–827Google Scholar
7. Kumar A, Jin Z, Bilker W, Udupa J, Gottlieb G: Late-onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci U S A 1998; 95:7654–7658Google Scholar
8. Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF III, Becker JT: Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry 2002; 159:1424–1427Google Scholar
9. Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A: Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry 2004; 161:99–108Google Scholar
10. Soares JC, Mann J: The anatomy of mood disorders: review of structural neuroimaging studies. Biol Psychiatry 1997; 41:86–106Google Scholar
11. Coffey CE, Figiel GS, Djang WT, Weiner RD: Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry 1990; 147:187–189Google Scholar
12. Thomas AJ, O’Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH: Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry 2002; 59:785–792Google Scholar
13. Hickie I, Scott E, Mitchell P, Wilhelm K, Austin MP, Bennett B: Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry 1995; 37:151–160Google Scholar
14. Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KRR: Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry 2004; 161:1293–1296Google Scholar
15. Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, Saito Y, Sawada S, Kinoshita T: Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 2006; 77:120–122Google Scholar
16. Nobuhara K, Okugawa G, Minami T, Takase K, Yoshida T, Yagyu T, Tajika A, Sugimoto T, Tamagaki C, Ikeda K, Sawada S, Kinoshita T: Effects of electroconvulsive therapy on frontal white matter in late-life depression: a diffusion tensor imaging study. Neuropsychobiology 2004; 50:48–53Google Scholar
17. Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO: Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry 2002; 159:1929–1932Google Scholar
18. Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD: Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry 2002; 51:342–344Google Scholar
19. Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO: White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry 2005; 162:602–605Google Scholar
20. Hao Y, Liu Z, Jiang T, Gong G, Liu H, Tan L, Kuang F, Xu L, Yi Y, Zhang Z: White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuroreport 2006; 17:23–26Google Scholar
21. Ashburner J, Friston KJ: Voxel-based morphometry: the methods. Neuroimage 2000; 11:805–821Google Scholar
22. Gitelman DR, Ashburner J, Friston KJ, Tyler LK, Price CJ: Voxel-based morphometry of herpes simplex encephalitis. Neuroimage 2001; 13:623–631Google Scholar
23. Basser PJ, Mattiello J, LeBihan D: Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994; 103:247–254Google Scholar
24. Basser PJ, Pierpaoli C: Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111:209–219Google Scholar
25. Rosenthal R, Rosnow RL: Essentials of Behavioral Research: Methods and Data Analysis, 2nd ed. New York, McGraw-Hill, 1991Google Scholar
26. Baxter LRJ, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM: Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46:243–250Google Scholar
27. Mayberg HS: Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 1997; 9:471–481Google Scholar
28. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675–682Google Scholar
29. Nofzinger EA, Buysse DJ, Germain A, Price JC, Meltzer CC, Miewald JM, Kupfer DJ: Alterations in regional cerebral glucose metabolism across waking and non-rapid eye movement sleep in depression. Arch Gen Psychiatry 2005; 62:387–396Google Scholar
30. Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML: A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 2005; 57:201–209Google Scholar
31. Biver F, Goldman S, Delvenne V, Luxen A, De Maertelaer V, Hubain P, Mendlewicz J, Lotstra F: Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry 1994; 36:381–388Google Scholar



